
Urothelial carcinoma, also known as transitional cell carcinoma (TCC), primarily affects the bladder but can also occur in the upper urinary tract (renal pelvis and ureters) and, less commonly, the urethra (1).
Urothelial carcinoma is cancer that starts in your urothelium, the tissue that lines parts of your urinary system.
Urothelial carcinoma accounts for about 90% of all cases of bladder cancer and 7% of all cases of kidney cancer, including cancer in your renal pelvis and ureter. Bladder and kidney cancers caused by urothelial carcinoma have similar symptoms. They also have similar prognoses — caught early on, these cancers are easily treated, but often come back (2).

Urothelial cancer, particularly bladder cancer, is a significant health concern in Saudi Arabia, with distinct epidemiological patterns influenced by age, gender, and various risk factors. The incidence of bladder cancer in Saudi Arabia has been studied extensively, with age-specific rates providing critical insights into disease distribution. According to data from the Saudi Cancer Registry, the overall incidence of bladder cancer was reported to be 1.4 per 100,000 persons (3). Urothelial carcinoma is the seventh most common cancer in men and the seventeenth in women globally. In the United States, it ranks as the sixth most common cancer overall. Approximately 75% of newly diagnosed cases are non-muscle invasive. Each year, around 110,500 men and 70,000 women in the European Union are diagnosed with this cancer, leading to approximately 38,200 deaths (4). According to the Saudi Cancer Registry’s 2013 report, urinary bladder cancer accounted for 4.3% of all newly diagnosed cancers in men and 0.8% in women, ranking it as the 8th most common cancer among males and 20th among females (5).
Though it can occur at any age, most people diagnosed with bladder cancer are older than 55 (6). However, the incidence increases significantly with age, a pattern consistent with many other cancers. Studies have shown that the highest incidence of bladder cancer occurs in individuals aged 61–80 years, with this age group accounting for 61.5% of cases in some regions (7). This age-related trend is further supported by global data, which indicate that bladder cancer incidence rises sharply in older populations (8,9).
Men are more likely to develop bladder cancer than women (6). Gender differences in bladder cancer incidence are pronounced, with men being disproportionately affected compared to women. In Saudi Arabia, the incidence of genitourinary cancer, which includes bladder cancer, is five-fold higher in men than in women (10). This gender disparity is consistent with global trends, where men are at higher risk due to factors such as higher rates of smoking and occupational exposures (11,12).
Smoking cigarettes, cigars or pipes may increase the risk of bladder cancer by causing harmful chemicals to accumulate in the urine. When you smoke, your body processes the chemicals in the smoke and excretes some of them in your urine. These harmful chemicals may damage the lining of your bladder, which can increase your risk of cancer (6). Smoking is the most well-established risk factor for bladder cancer in Saudi Arabia. The prevalence of smoking among Saudis is high, with 45.3% of males and 11.11% of females reporting current smoking habits (13). Cigarette smoking contributes to approximately 35–50% of bladder cancer cases globally, with the risk increasing significantly with the intensity and duration of smoking (14). A study conducted in Basrah found that current smokers had a nearly threefold increased risk of bladder cancer, while ex-smokers had a fourfold increased risk (15). The mechanisms underlying this association include the presence of carcinogenic compounds in tobacco smoke, such as polycyclic aromatic hydrocarbons (PAHs) and nitrosamines,which cause DNA damage and genetic mutations in bladder epithelial cells (14,16).
kidneys play a key role in filtering harmful chemicals from your bloodstream and moving them into your bladder. Because of this, it’s thought that being around certain chemicals may increase the risk of bladder cancer. Chemicals linked to bladder cancer risk include arsenic and chemicals used in the manufacture of dyes, rubber, leather, textiles and paint products (6).
Occupational exposure to carcinogens is another significant risk factor for bladder cancer in Saudi Arabia. Workers in industries involving aromatic amines, such as those in the chemical, dye, and rubber industries, are at higher risk (17,18). For example, exposure to PAHs in occupational settings has been linked to increased levels of oxidative stress and tumor markers, such as p53 and p21 proteins, which are associated with bladder cancer development (16).
A study in Makkah found that workers exposed to high levels of PAHs during the Hajj season had significantly elevated levels of malondialdehyde (MDA),a marker of oxidative stress, and tumor biomarkers compared to unexposed controls (16).
Treatment with the anti-cancer drug cyclophosphamide increases the risk of bladder cancer. People who received radiation treatments aimed at the pelvis for a previous cancer have a higher risk of developing bladder cancer (6).
Genetic predisposition also plays a role, with specific polymorphisms, such as rs1801133 and rs1801394, conferring susceptibility to urothelial bladder carcinoma in the Saudi population (19).

The pathogenesis of urothelial cancer is driven by a complex interplay of genetic mutations, environmental risk factors, and molecular pathway alterations. FGFR3 and TP53 mutations represent two distinct genetic pathways, while smoking and chemical exposure contribute to mutational signatures and epigenetic changes. Understanding these factors is crucial for developing targeted therapies and improving patient outcomes.
FGFR3 mutations are among the most frequent genetic alterations in urothelial cancer, particularly in non-muscle invasive bladder cancers (NMIBC). These mutations are associated with low- rade, low stage tumors and are often mutually exclusive with TP53 mutations. FGFR3 mutations activate signaling pathways such as the RAS/MAPK and PI3K/AKT pathways, promoting cell proliferation and survival (20).
TP53 mutations are more commonly observed in muscle-invasive bladder cancers (MIBC) and are associated with aggressive disease. TP53 mutations lead to the loss of tumor suppressor function, resulting in uncontrolled cell growth and genomic instability. These mutations are often linked to exposure to carcinogens such as those found in tobacco smoke and diesel exhaust (21,22).
Smoking is a well-established risk factor for urothelial cancer. Tobacco smoke contains carcinogens that induce genetic and epigenetic alterations. Smoking is associated with higher tumor mutation burden and specific mutational signatures, including ERCC2 and APOBEC signatures. Waterpipe smoking,a growing concern, also contributes to bladder cancer development, with similar genomic profiles to cigarette smoking (23,24).
Exposure to diesel exhaust and polycyclic aromatic hydrocarbons (PAHs) is another significant risk factor. Diesel exhaust contains nitrated PAHs (nitro-PAHs), which are potent mutagens. These compounds are associated with TP53 mutations and specific mutational signatures in bladder tumors, highlighting their role in carcinogenesis (21,22).
The PI3K/AKT pathway is frequently altered in urothelial cancer, often through mutations in PIK3CA, PTEN, and AKT1. Activation of this pathway promotes cell survival, proliferation, and invasion. Smoking and chemical exposure can induce epigenetic alterations, such as promoter methylation, which further activate this pathway (25,26,27).
The RAS/MAPK pathway is activated in urothelial cancer, particularly in low-grade tumors. Mutations in HRAS, KRAS, and NRAS are observed, although they are less frequent than FGFR3 mutations. The RAS/MAPK pathway is also influenced by environmental factors, such as smoking, which can induce specific mutational signatures (28,29).
The tumor microenvironment plays a critical role in urothelial cancer progression. Immune infiltration, particularly the presence of CD8+ T cells and B cells, is associated with prognosis. FGFR3 alterations are linked to immune infiltration and may influence responses to immunotherapy.
Additionally, the tumor microenvironment is shaped by environmental factors, such as smoking, which can modulate immune cell activity (30).
• Epigenetic changes, such as DNA methylation, are common in urothelial cancer. Smoking and chemical exposure can induce hypermethylation of tumor suppressor genes, such as MCAM, DCC, and HIC1. These alterations contribute to tumorigenesis and progression. Epigenetic changes are also associated with specific molecular pathways, such as the PI3K/AKT pathway, and can serve as biomarkers for prognosis and treatment response (30,31,32).
Urothelial cancer encompasses malignancies that arise from the urothelium, the tissue lining the urinary tract, including the bladder, ureters, renal pelvis, and urethra. These cancers are primarily classified based on their location, with bladder urothelial cancer being the most prevalent, followed by cancers of the upper urinary tract, which includes the renal pelvis and ureters, and finally, urethral urothelial cancer.
Bladder cancer is the most common form of urothelial cancer, accounting for 90-95% of cases (33). It is often misdiagnosed as urinary tract infections or nephrolithiasis, leading to delayed treatment (34). Approximately 54,000 cases are diagnosed annually in the United States (35). In Saudi Arabia, bladder cancer is more common in males, with a male-to-female ratio of approximately 4.4:1. The majority of cases are diagnosed in individuals over the age of 60 (36).
UUTUCC includes cancers of the renal pelvis and ureters, representing about 5-10% of urothelial cancers (33). These tumors are more common in the renal pelvis than in the ureters (33). Risk factors include smoking, chronic urinary tract infections, and previous bladder cancer (33). UUTUCCs are often invasive at diagnosis, with a lower survival rate compared to superficial tumors (33). Specific incidence rates for UTUC in Saudi Arabia are not well-documented, indicating a need for more focused research in this area.
Urethral cancer is less common and often shares diagnostic and treatment challenges with other urothelial cancers (34). It is frequently grouped with other urothelial neoplasms in statistical analyses, making specific incidence data scarce (34). This is the rarest form of urothelial carcinoma. Data on urethral cancer incidence in Saudi Arabia are scarce, reflecting its infrequent occurrence.
Urothelial cancer, primarily affecting the bladder, presents with a variety of symptoms that can significantly impact patients’ quality of life. These symptoms can vary in intensity and may not all be present in every patient. The presence and severity of symptoms often depend on the stage of the cancer and whether it has metastasized to other parts of the body. Early detection and treatment are crucial for managing symptoms and improving quality of life for patients with urothelial cancer. Below are the detailed symptoms associated with this type of cancer:
The most common symptom of urothelial carcinoma is hematuria, which is the presence of blood in the urine. This symptom is often painless and can be either microscopic or macroscopic (37,38,39).
Patients may experience increased urinary frequency, urgency, and dysuria (painful urination). These symptoms can mimic those of a urinary tract infection or overactive bladder syndrome, sometimes leading to a delay in diagnosis (38,39).
Pain is a prevalent symptom, often manifesting as lower back pain on one side of the body or abdominal pain. This can be due to the tumor’s location or its spread to adjacent structures (37,38).
Fatigue is a common symptom reported by patients undergoing treatment for urothelial cancer. This can be exacerbated by the cancer itself or as a side effect of treatments like chemotherapy or immunotherapy (40,41).
Some patients report swelling of the arms or legs, which can be a side effect of treatment or due to the cancer’s progression (40).
In cases where the cancer has metastasized, patients may experience pulmonary symptoms such as pleural effusion, pulmonary embolism, or other respiratory issues (39).
Although rare, some patients with urothelial carcinoma may develop PNS, which includes neurological symptoms like cerebellar syndromes and encephalomyelitis (42).

Physical examination plays a limited but supportive role in the diagnosis of urothelial cancer. The most common presenting symptom is painless hematuria, which warrants further investigation (44). Physical examination may include:
To detect any palpable masses or tenderness in the abdominal region, which could indicate advanced disease or metastasis (45,46).
In women, to assess for any signs of local spread to the pelvic organs.
In men, to evaluate the prostate for signs of involvement by urothelial carcinoma, particularly in the prostatic urethra (44).
While physical examination is not definitive, it helps in identifying high-risk patients who require further diagnostic workup.
Laboratory tests are integral to the diagnosis and monitoring of urothelial cancer. Key methods include:
A test to check the color of urine and its contents, such as sugar, protein, blood and bacteria (47).
The presence of blood in urine is the most common symptom of urothelial cancer. However, hematuria can also result from benign conditions such as urinary tract infections or kidney stones (44).
This involves urine microscopical examination for abnormal cells. While it has high specificity, its sensitivity is limited, particularly for low-grade tumors (48). Cancer in your kidneys, bladder or ureter may shed cancer cells into your urine (47).
Urine cytology is particularly useful for monitoring recurrence in previously diagnosed patients.
Laboratory tests are integral to the diagnosis and monitoring of urothelial cancer.
A tumor marker that is elevated in urothelial cancer. It is used for surveillance but has limited sensitivity for early detection (49).
Detects chromosomal abnormalities associated with urothelial cancer. It is more sensitive than cytology but less specific (49).
A DNA-based test detecting alterations in TERT, FGFR3, and KRAS genes. It has shown high sensitivity (80.2%) and specificity (96.9%) in detecting non-muscle-invasive bladder cancer (NMIBC) (50).
A multitarget urine DNA test that integrates FGFR3 and TERT mutations with methylation markers. It demonstrates a sensitivity of 91.37% and specificity of 95.09% for urothelial carcinoma detection (51).
Emerging biomarkers such as circulating tumor DNA (ctDNA) and extracellular vesicles are being explored for their potential in non-invasive diagnosis and monitoring of urothelial cancer (49).
Imaging plays a crucial role in the staging and surveillance of urothelial cancer. The choice of modality depends on the tumor location (upper vs. lower urinary tract) and the clinical context. Imaging studies are essential for visualizing the urinary tract and identifying tumors. Techniques such as ultrasound, CT scans, and MRI are commonly used to assess the presence and extent of urothelial cancer (54,55). These imaging modalities help in staging the cancer and planning treatment strategies.
1.3.Retrograde Pyelography
Complementary imaging for suspected lesions when radiological findings are inconclusive (54).
2. Lower Urinary Tract Urothelial Carcinoma
2.1.Ultrasound
A procedure in which high-energy sound waves (ultrasound) are bounced off internal tissues or organs and make echoes. The echoes form a picture of body tissues called a sonogram. Healthcare providers may do an abdominal ultrasound to help diagnose cancer of your renal pelvis and ureter (47). Limited in staging accuracy but useful for initial assessment of hematuria (55).
2.2.CT and MRI
Provide detailed information on tumor invasion depth and lymph node involvement. CT is preferred for its higher sensitivity (44).
2.3.PET scan
Emerging as atool for detecting metastatic disease, particularly in advanced cases (53).
3. Surveillance Imaging
3.1.CT urogram
The workhorse for surveillance of both upper and lower tract urothelial cancer (44,52).
3.2.MRI
Used for long-term follow-up to minimize radiation exposure (53).
4. Intravenous pyelogram (IVP)
A series of X-rays of your kidneys, ureter and bladder to check for cancer. Healthcare providers inject a contrast dye into one of your veins. Then, they take X-rays as the dye moves through your kidneys, ureter and bladder to see if there are any blockages (47).
Endoscopy is the cornerstone of urothelial cancer diagnosis, particularly for bladder cancer. Cystoscopy is the gold standard for diagnosing urothelial cancer, allowing direct visualization of the bladder and urethra. During cystoscopy, any abnormal tissue can be biopsied for further analysis (56,57). This procedure is invasive but provides critical information about the presence and characteristics of tumors.
1. Cytoscopy
1.1.White Light Cystoscopy (WLC)
The current gold standard for detecting bladder tumors. It involves direct visualization of the bladder mucosa under white light (58.59).
1.2.Blue Light Cystoscopy (BLC)
Enhances the detection of flat lesions and carcinoma in situ (CIS) by using a photosensitizing agent that fluoresces under blue light (44).
1.3.Narrow Band Imaging (NBI)
Improves visualization of tumor vasculature, aiding in differentiating malignant from benign lesions (59).
2. Ureterorenoscopy (URS)
Providers use a thin tube-like instrument with a light and lens for viewing to look inside your ureter and renal pelvis and to take tissue samples (47). Used for diagnosing upper tract urothelial carcinoma. It allows direct visualization and biopsy of suspicious lesions in the ureters and renal pelvis (52,54).
3. Biopsy and Transurethral Resection of Bladder Tumor (TURBT)
3.1.Biopsy
Essential for histopathological confirmation of malignancy. It is performed during cystoscopy or ureterorenoscopy (58,60).
3.2.TURBT
Atherapeutic and diagnostic procedure where the tumor is resected for histopathological analysis (61).
Histopathology is the definitive diagnostic tool for urothelial cancer. Histopathological examination of biopsy samples is crucial for confirming the diagnosis of urothelial cancer. It involves analyzing the tissue under a microscope to determine the type, grade, and stage of the cancer (57,62). This information is vital for developing an appropriate treatment plan and predicting the prognosis.
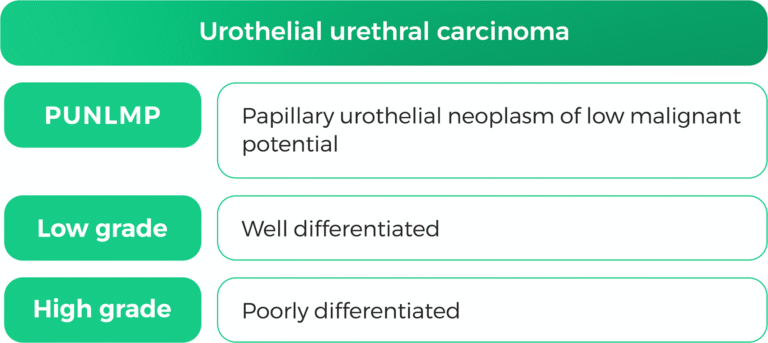
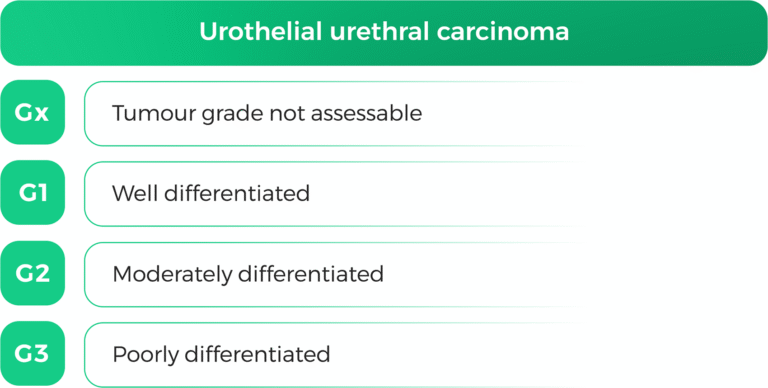
1. Histological Classification
Urothelial carcinoma is classified into non-muscle-invasive (NMIBC) and muscle-invasive (MIBC) types based on tumor invasion depth (63). The 2022 WHO classification recognizes over 13 subtypes of urothelial carcinoma, each with distinct cytomorphologic features (64).
2. Cytological Evaluation
Urine cytology is used for initial screening and surveillance. However, its sensitivity is lower for low-grade tumors (58). Cell block preparations and immunohistochemistry can enhance diagnostic accuracy in challenging cases (64).
3. Molecular Pathology
Emerging techniques such as next-generation sequencing are being explored to identify molecular alterations for personalized treatment strategies (49).
1.1.Photodynamic Diagnosis (PDD)
Uses photosensitizing agents to enhance tumor visibility during cystoscopy (59).
1.2.Confocal Laser Endomicroscopy (CLE)
Provides real-time histological imaging during endoscopy, enabling optical biopsies (59).
1.3.Optical Coherence Tomography (OCT)
Offers subsurface imaging to assess tumor depth and staging (59).
AI-based software is being developed to enhance the interpretation of endoscopic images and improve diagnostic accuracy (59).
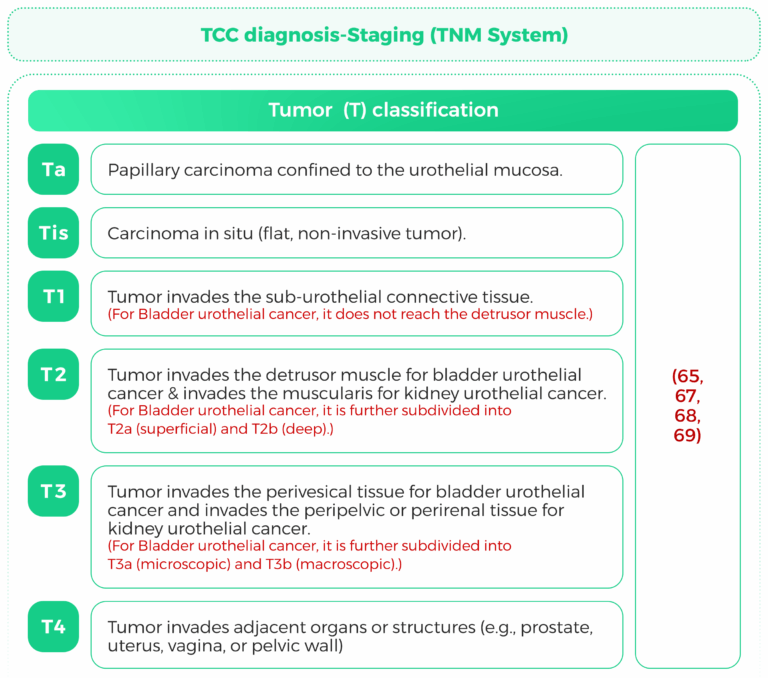
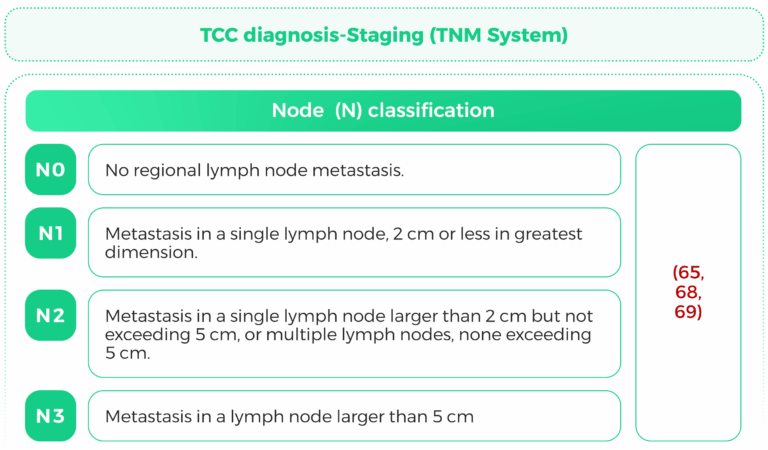
The TNM (Tumor, Node, Metastasis) staging system is a widely used classification system for cancer staging. It provides critical information about the extent of tumor spread, which is essential for prognosis and treatment planning. This response focuses on the TNM staging criteria for kidney urothelial cancer, bladder urothelial cancer, and urothelial carcinoma, with a particular emphasis on tumor size and lymph node involvement.
Once diagnosed, staging is important to establish treatment. The TNM system (Tumor, Node,Metastasis) is utilized:
T (Extent of tumor): Indicates the size and extent of the primary tumor into nearby tissues.
N (Nodeinvolvement): Involvement of regional lymph nodes.
M (Metastasis): Remote organ involvement.
Other Staging Tools:
Lymph Node Biopsy (Fine Needle Aspiration- FNA): In case of swollen nodes.
Bone scan: In the suspicion of bone metastasis.
For urothelial cancers, including kidney, bladder, and other urothelial cancers, the TNM system is applied to determine the stage of the disease, which guides treatment decisions and predicts outcomes (65,66).
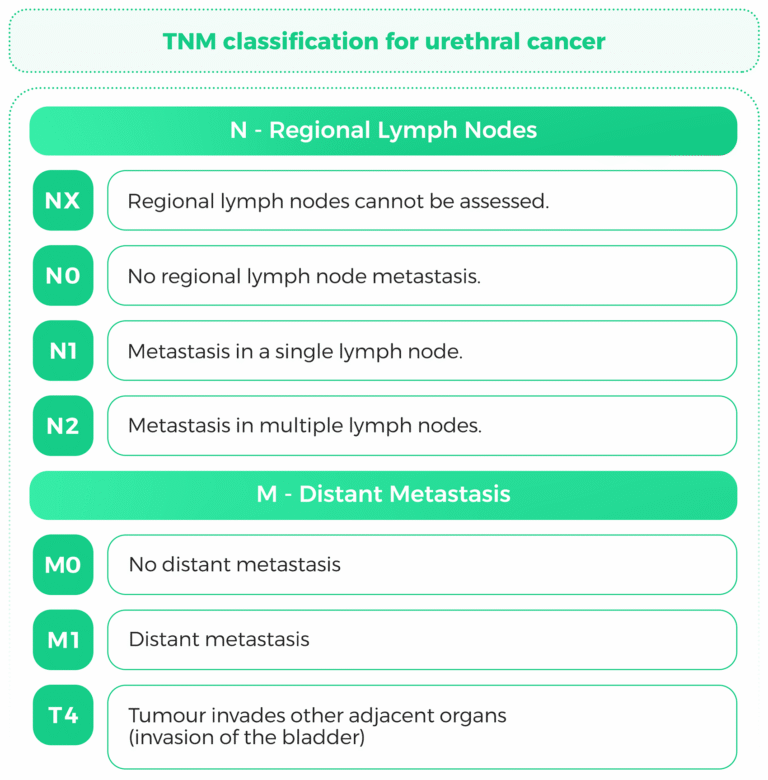

Lymph node involvement is a critical prognostic factor in bladder cancer. Studies have shown that patients with lymph node metastasis (N+) have significantly worse overall survival (OS) and cancer specific survival (CSS) compared to those without lymph node involvement (N0) (70,71).
Lymph node status is a key prognostic factor for kidney urothelial cancer. Patients with lymph node metastasis have a higher risk of disease recurrence and cancer-specific mortality. The extent of lymph node dissection during surgery has been shown to improve survival outcomes, particularly in patients with high-risk disease (72,73).
Lymphnode involvement is a significant prognostic factor for urothelial carcinoma. The lymph node ratio (LNR), defined as the ratio of positive lymph nodes to the total number of lymph nodes removed, has been identified as an independent predictor of survival. A higher LNR is associated with worse overall survival and cancer-specific survival (71,74).

Preventing urothelial cancer involves a multifaceted approach that includes lifestyle modifications, chemoprevention, and medical interventions. Primary prevention strategies focus on reducing exposure to known risk factors, while secondary and tertiary prevention aim to detect early disease and prevent recurrence.
Cigarette smoking is a significant risk factor for urothelial cancer, increasing the risk threefold. Smoking cessation is a powerful tool in reducing the incidence of this cancer (75,76).
A diet rich in fruits, vegetables, and certain nutrients like selenium and folic acid may lower the risk, especially in smokers. High fluid intake is also recommended to reduce risk (76).
This compound, derived from the kava plant, has shown promise in reducing tumor burden and improving survival in animal models of bladder cancer by targeting the Ha-ras pathway (77).
Retinoids, difluoromethylornithine, and NSAIDs have been explored for their potential in tertiary prevention, particularly after surgical interventions (76,78).
This is a form of tertiary prevention where chemotherapy or immunotherapy is directly administered into the bladder to prevent recurrence after tumor resection (78).
Identifying biomarkers for early detection and monitoring treatment response is crucial for secondary prevention. This includes urinary cytokines and genetic markers (78).
Treatment approaches vary based on several factors:
The treatment of urothelial cancer is influenced by a variety of factors, including patient characteristics, tumorbiology, and available therapeutic options. Here are the key factors affecting the treatment of urothelial cancer:
1.1. Age and Comorbidities
Older age and higher comorbidity burden often lead to the selection of immunotherapy over chemotherapy due to bettert olerance (79).
1.2. Performance Status
Patients with better performance status are more likely to receive aggressive treatments, including combinationtherapies (80).
2.1. Histology and Stage
The presence of variant histologies, such as micropapillary features, and the stage of the tumor significantly influence treatment decisions. High-risk tumors often require radical treatments like nephroureterectomy (80,81).
2.2. Genetic Mutations
High tumor mutational burden and specific mutations, such as FGFR3 alterations, can guide the use of targeted therapies like erdafitinib (82).
3.1. Drug Resistance
Intrinsic and acquired resistance to chemotherapy and immunotherapy remains a significant challenge, necessitating the development of novel therapeutic strategies (83).
3.2. Therapy Attrition
Many patients do not progress beyond first-line therapy due to disease progression or treatment-related toxicity, impacting overall survival outcomes (84).
4.1. Chemotherapy
Platinum-based chemotherapy remains a standard first-line treatment, but its use is limited by patient eligibility and potential side effects (82,84).
4.2. Immunotherapy
PD-1/PD-L1 inhibitors are used, especially in patients ineligible for cisplatin, and have shown improved survival in some cases (79,82).
4.3. Combination Therapies
Combination chemo-immunotherapy is more frequently used in academic centers and has been associated with improved survival compared to chemotherapy alone (79).
Urothelial cancer, which primarily affects the bladder and upper urinary tract, has seen significant advancements in surgical techniques and technological innovations. These advancements aim to improve treatment efficacy, reduce morbidity, and enhance patient outcomes. This response provides acomprehensive overview of the current state of surgical techniques and technological advancements in the treatment of urothelial cancer, citing relevant research papers.
1.1.1. Contemporary TURBT Techniques
Transurethral resection of bladder tumors (TURBT) remains the gold standard for diagnosing and treating non-muscle-invasive bladder cancer (NMIBC). Recent advancements include en bloc resection, bipolar electrocautery, and laser resection, which improve tumor excision rates and reduce recurrence (85,86,87).
1.1.2. Enhanced Endoscopic Imaging
Technologies such as narrow-band imaging (NBI), photodynamic diagnosis (PDD), and optical coherence tomography (OCT) have enhanced the detection and staging of bladder tumors. These tools improve the sensitivity of tumor detection and reduce the risk of missing lesions during TURBT (88,89).
1.1.3. Image-Guided Surgery
Image-guided TURBT, including blue light cystoscopy (BLC) and molecular imaging agents, has further improved the quality of resections. These technologies enable real-time visualization of tumors, aiding in more thorough resection and reducing the likelihood of residual cancer (89,90).
1.2.1. Laparoscopic and Robotic-Assisted Extended Pelvic Lymph Node Dissection (PLND)
Minimally invasive techniques, including laparoscopic and robotic-assisted extended PLND, have become integral to the management of invasive bladder cancer. These approac hes offer benefits suchas minimal invasiveness, superior visualization, and reduced blood loss, while adhering to the principles of open surgery (91,92).
1.2.2. Nephron-Sparing Surgery (NSS)
For patients with low-risk upper tract urothelial carcinoma, nephron-sparing surgery (NSS) is preferred. Advances in diagnostic tools, lasers, and topical chemotherapy have optimized NSS outcomes, reducing tumor recurrence and progression (93,94).
1.2.3. Bladder-Sparing Approaches
Bladder-sparing approaches, combining transurethral debulking surgery with chemotherapy and radiation, have shown promising results. These strategies aim to preserve bladder function while achieving high complete response rates (88%) and low distant metastasis rates (90%) (94).
1.3.1. Robot-Assisted Radical Cystectomy (RARC)
Robot-assisted radical cystectomy (RARC) has emerged as a modern and effective treatment for muscle-invasive bladder cancer (MIBC). Studies have demonstrated that RARC offers improved surgical precision, reduced blood loss, and faster recovery times compared to traditional open and laparoscopic methods (95,96). The use of intraoperative indocyanine green (ICG) fluorescence during RARC has further enhanced the accuracy of lymph node dissection and tumor identification, with highsensiti vity (94.4%) and specificity (83.3%) reported (95).
1.3.2. Robotic Radical Nephroureterectomy (RNU)
For upper tract urothelial carcinoma (UTUC), robotic radical nephroureterectomy (RNU) with bladder cuff excision has become a preferred approach. This technique, refined over more than 150 cases, incorporates regional lymphadenectomy and intraoperative instillation of intravesical chemotherapy, offering a standardized and efficient management strategy for high-grade and bulky tumors (92,97).
Advantages of Robotic Surgery The robotic platform provides seven degrees of freedom, 10× magnification, and a three-dimensional view, enabling precise dissection and minimizing complications. These technological advancements have made robotic surgery a viable alternative to open and laparoscopic techniques, with comparable oncological outcomes (98).
Near-Infrared Fluorescence Imaging
The use of near-infrared fluorescence imaging with agents like ICG has revolutionized intraoperative visualization of tumors and lymphatic vessels. This technology enables personalized surgical planning and reduces postoperative complications (90).
2.Radiotherapy
Radiation therapy for urothelial cancer, particularly in cases where surgery is not feasible, has shown promise as a treatment option. This approach is especially relevant for patients with nonmetastatic upper-tract urothelial carcinoma (UTUC) who are medically inoperable. Studies have demonstrated that radiotherapy can achieve good local tumor control with manageable side effects, making it a viable alternative for certain patient populations. The following sections provide a detailed overview of the findings from recent research on this topic.
2.1. Efficacy of Radiation Therapy in Non metastatic UTUC
Radiotherapy has been effective in achieving local tumor control in patients with nonmetastatic UTUC who are not candidates for surgery. In a study involving eight patients, all achieved local control, although two developed distant metastases and succumbed to tumor progression (99). Stereotactic Ablative Radiation Therapy (SABR) has also been shown to be safe and effective, with most patients maintaining stable renal function and experiencing minimal acute side effects (100).
2.2. Role of Stereotactic Body Radiation Therapy (SBRT) in Metastatic Urothelial Cancer
SBRT has been beneficial for patients with oligometastatic urothelial cancer, achieving local control in a significant percentage of treated lesions. This approach can delay the need for systemic treatments and improve progression-free survival (101,102). In a retrospective study, SBRT provided effective local control with minimal adverse events, suggesting it as a safe option for selected patients with oligometastatic disease (102).
2.3. Palliative Use of Radiotherapy in Metastatic Urothelial Carcinoma
Radiotherapy serves a palliative role in metastatic urothelial carcinoma, helping to alleviate symptoms and improve quality of life. It can be used a long side systemic therapies to enhance patient outcomes(103).
While radiation therapy offers a promising alternative for certain urothelial cancer patients, it is important to consider the potential for distant metastases and the need for careful patient selection. Further research is needed to refine treatment protocols and identify optimal candidates for this approach.
3. Immunotherapy
Immunotherapy has emerged as a promising treatment for urethral cancer, particularly in advanced stages. Recent studies highlight the effectiveness of immune checkpoint inhibitors (ICIs) combined with chemotherapy, demonstrating improved overall survival (OS) and progression-free survival (PFS) compared to traditional platinum-based chemotherapy alone.
3.1.Immune checkpoint inhibitors
3.1.1. Pembrolizumab
Pembrolizumab is a monoclonal antibody that functions as an immune checkpoint inhibitor bytargeting the PD-1 receptor on lymphocytes. By blocking the PD-1/PD-L1 interaction, it restores T-cell activity against tumors and enhances the immune response to cancer cells. Pembrolizumab stimulates
both innate and adaptive immune cells, promoting cytotoxic phenotypes and increasing cytokine secretion. The standard dose used is 200 mg administered intravenously every three weeks or 400 mg every 6 weeks (104,105,106,107,108,110,111).
Side effects(106,107,109) .
3.1.2. Nivolumab
Another PD-1 inhibitor, nivolumab, enhances T-cell responses against tumors by blocking PD-1 (112).
Nivolumab is given through intravenous infusion (IV), with a standard dose of 240 mg every 2 weeks (113) or 480 mg every 4 weeks (110). The side effects of nivolumab include rash, fatigue, and the potential for severe immune-mediated adverse effects (114).
3.1.3. Atezolizumab
Atezolizumab is a PD-L1 inhibitor that prevents PD-L1 from binding to PD-1 and B7.1, promoting T cell activation (112). Atezolizumab is administered intravenously at 1200 mg every 3 weeks (113) or 840 mg every 2 weeks (110).
3.1.4. Avelumab or Durvalumab
They are a PD-L1 inhibitor that enhances T-cell responses and anti-tumor immunity by blocking PD L1 interactions (112).
Avelumab is given intravenously at 800 mg every 2 weeks (113).
Durvalumab is commonly dosed at 1500 mg every 4 weeks intravenously (113).
Nivolumab, atezolizumab, avelumab, and durvalumab share the same adverse effects, including rash, fatigue, and potential for severe immune-mediated adverse effects (114).
3.2. Erdafitinib
Erdafitinib is an oral pan-fibroblast growth factor receptor (FGFR) inhibitor approved for treating locally advanced or metastatic urothelial carcinoma (mUC) in patients with specific FGFR alterations (115,116).
Erdafitinib inhibits FGFR signaling pathways by inhibiting their tyrosine kinase activity, which are crucial for tumor growth and survival in FGFR-altered cancers (115,116).
Erdafitinib is administered orally, allowing for convenient outpatient treatment (115).
The initial dose is 8 mg once daily, possibly up titration to 9 mg based on individual tolerance and response (118).
Common side effects include hyperphosphatemia, fatigue, and gastrointestinal disturbances.
Serious adverse events (grade 3 or 4) occurred in approximately 45.9% of patients, with a lower incidence of treatment-related deaths compared to chemotherapy (116,117).
4. Antibody-drugconjugate
Antibody-drug conjugates (ADCs) have emerged as a significant advancement in the treatment of urethral cancer, particularly urothelial carcinoma.
The primary ADCs currently utilized include enfortumab vedotin, Disitamab vedotin, and sacituzumab govitecan.
Each agent operates through distinct mechanisms, dosing regimens, and side effect profiles, which are crucial for clinical application.
4.1. Sacituzumab govitecan (SG):
Targets Trop-2 with SN-38 as the cytotoxic payload. SG is given at 10 mg/kg intravenously on days 1 and 8 of a 21-day cycle. It is also associated with several side effects, including gastrointestinal symptoms and neutropenia (119).
4.2. Enfortumab vedotin (EV):
4.3. Disitamab vedotin (DV):
(a promising treatment for urethral c ancer, especially in HER-2expressing tumors).
Disitamab vedotin (DV) is an HER-2-targeted monoclonal antibody linked to a cytotoxic drug—monomethyl auristatin E (MMAE)—which disrupts microtubules and induces cell death (121,122).
The DV dose regimen varies depending on the treatment protocol, but it is commonly administered intravenously at 2 mg/kg doses every 2 weeks (123,124). DV is associated with many adverse effects that vary in severity, including nausea, alopecia, diarrhea, tachycardia, anorexia, peripheral sensory neuropathy, and fatigue (122,123,124).
5.Immunotherapies combinations
5.1. ICIs and ADC combinations
The combination of enfortumab vedotin and pembrolizumab has emerged as a standard first-line therapy for locally advanced or metastatic UC. This combination achieved a 53% reduction in the risk of death compared to chemotherapy in the EV-302 trial. This combination is given as first-line therapy, with enfortumab vedotin at 1.25 mg/kg on days 1 and 8 and pembrolizumab at 200 mg every 3 weeks. However, The combination of ICIs and ADCs may lead toincreased toxicity due to the synergistic effects of both agents. However, clinical trials have shown that these combinations are tolerable with appropriate monitoring (125,126).
5.2. Targeted Therapies and ICIs
Targeted therapies, such as erdafitinib (an FGFR inhibitor), have shown efficacy when combined with ICIs in FGFR-altered UC. Ongoing trials are exploring the potential of such combinations in the metastatic setting (127).
Predictive Biomarkers and Patient Selection
1. PD-L1 Expression
PD-L1 expression is a widely used biomarker to predict response to ICIs. However, its utility is limited due to variability in expression and assay standards (128,129).
2. Tumor Mutational Burden (TMB)
High TMB has been associated with improved response to ICIs in UC. Emerging data suggest that TMB may complement PD-L1 expression in patient selection (129,130).
3. Novel biomarkers
A novel RNA-based ICI response signature (ICI-PRS) has been developed to predict response to ICIs
This signature integrates tumor-derived and immune-associated features to identify patients most likely to benefit from immunotherapy (129).
Emerging Trends and Future Directions
1. Next-Generation ICIs
Research is ongoing to develop next-generation ICIs with improved efficacy and reduced toxicity. Bispecific antibodies and cytokine-based therapies are being explored in early clinical trials (130,131).
2. Personalized Medicine
The integration of biomarkers, such as ICI-PRS and TMB, into clinical practice is expected to enableperso nalized treatment strategies for UC patients (129,130).
3. Combination Therapies
The exploration of combination therapies involving ICIs, ADCs, and targeted agents is a promising area of research. These combinations aim to overcome resistance and enhance therapeutic efficacy in UC (125,126,127).
6. Chemotherapy
6.1.Cisplatin-based regimen (most common):
Cisplatin is a fundamental chemotherapy used in urothelial cancer management. It crosslinks inter- and intra-DNA purine bases, which interferes with DNA replication, transcription, and repair mechanisms, leading to DNA damage and cancer cell apoptosis (132,122,134).
Cisplatin is usually delivered intravenously, allowing for direct bloodstream entrance and rapid distribution at 70 mg/ m2 on day 1 of a 3-week cycle (133,135,136).
For patients suffering from renal impairment, the dose regimen has to be split (e.g., 35 mg/m2 on day 1 and day 8) to reduce cisplatin-induced toxicity, especially nephrotoxicity (135,136). Various adverse effects differ in severity and are associated with cisplatin administration, ranging from nephrotoxicity, gastrointestinal disorders, hematologic toxicity, and decreased immune response to more severe conditions, such as acute kidney injury and hearing loss, which are significant concerns, especially in younger patients (133,134)
Resistance can develop, often due to enhanced DNA repair mechanisms in cancer cells, necessitating combination therapies to improve outcomes (132,139). Hence, its combination with gemcitabine and chemoradiation regimens for enhanced efficacy (137,138).
6.2.Mitomycin and mitomycin-C
Mitomycin and mitomycin-C (MMC) are chemotherapeutic agents that are effective in treating urothelial cancer because they can prevent recurrence and progression when used as adjuvant therapies.
Mitomycin-C has a dual mechanism of action direct cytotoxicity and immune system activation—making MMC a valuable treatment option. MMC is an antibiotic that functions as an alkylatingagent, causing the cross-linking of DNA and inhibiting both DNA synthesis and cell division, leading to cell death. Additionally, MMC induces immunogenic cell death (ICD), which enhances the immune system’s ability to recognize and target cancer cells, potentially reducing recurrence rates (140,141).
The choice of dosage and route depends on the tumor’s location, stage, and the patient’s overall health. The most common route of administration is intravesical installation, allowing for localizedtreatment with minimal systemic toxicity (142,143). MMC (40 mg) is diluted in 50 mL or 80 cc saline, held in the urethra for 30 minutes, and repeated weekly for 6 weeks (142,134).
MMC can be administered intravenously (IV) for advanced or metastatic cancers, providing systemic exposure to the drug. However, it is associated with higher toxicity. MMC (20 mg) is administered IV with a median of 6 cycles (range 2–12 cycles) and a cumulative dose of 120 mg (144).
Furthermore, the MMC (0.1%) solution is injected directly submucosally into the tumor site during urethrotomy, ensuring high concentrations of the drug at the target site and reducing the risk of recurrence (145,146).
While mitomycin-C is generally well-tolerated when used intravesically, it can cause several adverse effects, including local toxicity such as dysuria (painful urination) and increased frequency of urination (147). Some patients reported urethral strictures, particularly after intralesional injections (142). When MMC is administered IV, systemic toxicity is more common, such as hematological toxicity, including thrombocytopenia, leukopenia, and anemia (144). Other adverse effects, including nausea, fatigue, and allergic reactions, are less frequently reported but can occur with both intravesical and systemic administration (144,147).
6.3. Doxorubicin
Doxorubicin is a widely used chemotherapeutic agent for treating various cancers, including urothelial cancer, due to its multifaceted mechanisms of action. Doxorubicin intercalates with DNA, disrupting the function of topoisomerase II, which is crucial for DNA replication and transcription(148). It also induces apoptosis through the activation of the Bcl-2/Bax pathway and caspase activation (149). Additionally, it generates reactive oxygen species (ROS), which contribute to its cytotoxic effects (150).
Doxorubicin is commonly administered intravesically for urothelial cancer, with a dose around 1 mg/mL (141). Dox can also be administered via a continuous infusion at doses ranging from 125 to 2,500 mg over multiple cycles (151).
Despite its efficacy, doxorubicin is associated with significant adverse effects, including cardiotoxicity, hepatotoxicity, and testicular toxicity, which are dose-limiting factors (153).
Intravesical administration can lead to enhanced bladder contractile activity and sensory nerve activity, causing urological side effects (151).
Combining doxorubicin with other agents or protective pharmacological remedies is being explored to enhance efficacy and reduce toxicity (153). Efforts to mitigate doxorubicin’s adverse effects include the development of novel drug delivery systems, such as liposomes and nanoparticles (149).
6.4. Epirubicin
Epirubicin is an anthracycline antibiotic that has been widely used in the treatment of various cancers, including urothelial cancer.
Epirubicin functions primarily as an intercalating agent, inserting itself between DNA base pairs and inhibiting the synthesis of macromolecules, particularly DNA and RNA. This interference disrupts the replication of cancer cells and induces apoptosis, a form of programmed cell death. It also generates free radicals, which further damage cellular components, including DNA, proteins, and cell membranes (154,155). In addition to its direct cytotoxic effects, epirubicin enhances the uptake and nuclear translocation of other chemotherapeutic agents when used in combination, thereby increasing their efficacy. For instance, when combined with alpha1-oleate, epirubicin’s nuclear translocation is enhanced, leading to greater antitumor effects (156).
Epirubicin is commonly administered via intravesical instillation for the treatment of superficial bladder and urethral cancers. This method involves the direct delivery of the drug into the bladder through a catheter, allowing for high concentrations of the drug to come into contact with the tumor cells while minimizing systemic exposure and toxicity. Intravesical administration is typically performed in an outpatient setting and is well-tolerated by most patients (157,158,159).
Theadverse effects of epirubicin vary depending on the route of administration. When administered intravesically, the most common side effects are localized to the bladder and include cystitis, dysuria (painful urination), and pollakiuria (frequent urination). These effects are generally mild and transient, resolving shortly after treatment cessation (157,158,155).
Systemic administration of epirubicin is associated with more severe side effects, including myelosuppression (reduction in blood cell production), cardiotoxicity, alopecia (hair loss), and gastrointestinal disturbances such as nausea and vomiting. Myelosuppression can lead to neutropenia, anemia, and thrombocytopenia, which increase the risk of infections and bleeding.
Cardiotoxicity, a well-known side effect of anthracyclines, can lead to congestive heart failure, particularly with higher cumulative doses (155,160,161).To mitigate the risks associated with epirubicin, careful patient selection and close monitoring are essential. For intravesical therapy, patients are advised to drink plenty of fluids to help flush out the drug and reduce the risk of bladder irritation. Systemic administration requires regular monitoring of blood counts and cardiac function. In cases of severe toxicity, dose reduction or discontinuation of the drug may be necessary (155,157,158).
Epirubicin in combination with other treatments
Epirubicin is often used in combination with other therapeutic agents to enhance its efficacy and address various aspects of cancer treatment. Below are some common combinations:
1. Intravesical Combinations
Epirubicin is frequently combined with other intravesical agents, such as Bacillus Calmette-Guérin (BCG) and alpha2b-interferon (alpha2b-IFN), to improve the prophylaxis of recurrence in superficial bladder cancer. These combinations have been shown to reduce recurrence rates and extend disease
free survival. For example, the combination of epirubicin and BCG has been associated with a lower recurrence rate compared to monotherapy with either agent alone (162,163).
2.Systemic Combinations
Inadvanced or metastatic urothelial cancer, epirubicin is often used as part of multi-drug regimens. For instance, the MEC regimen (methotrexate, epirubicin, cisplatin) and the PEM regimen (cisplatin, epirubicin, methotrexate) have demonstrated significant antitumor activity, with response rates
ranging from 47% to 57%. These combinations are particularly effective in patients with locally advanced or metastatic disease (160,164,165,161).
Epirubicin is often used in combination with other therapeutic agents to enhance its efficacy and address various aspects of cancer treatment. Below are some common combinations:
3.Experimental combination
Recent studies have explored the potential of combining epirubicin with newer agents, such as paclitaxel, to enhance its efficacy in second-line chemotherapy for metastatic urothelial carcinoma. The combination of epirubicin and paclitaxel has shown promising results, with a disease control
rate of 52% and manageable toxicity (160).
6.5. Paclitaxel
Paclitaxel has been extensively studied in urothelial carcinoma. Paclitaxel is a versatile chemotherapeutic agent with a well-established mechanism of action and multiple routes of administration (166).
Paclitaxel, a diterpene taxane, exerts its anticancer effects by stabilizing microtubules, thereby preventing their disassembly. This stabilization disrupts the normal dynamics of the microtubule network, leading to cell cycle arrest at the G2/M phase and ultimately inducing apoptosis in cancer cells (167,168).
Paclitaxel can be administered through various routes, each offering distinct advantages. Intravenous (IV) Administration is the most common route, particularly for systemic treatment of advanced cancers. IV administration ensures rapid drug delivery and high bioavailability (167,169). The dose range varies between 70 mg/m² to 300 mg/m², administered every 3-4 weeks. For example, a study using a Cremophor-free polymeric micelle formulation administered 240 mg/m² every 3 weeks, with potential escalation to 300 mg/m² if well-tolerated (169,170).
Intravesical Administration involves direct instillation of paclitaxel into the bladder for localized treatment of urothelial cancers. It minimizes systemic toxicity and allows for higher drug concentrations at the tumor site (171).
Nanoparticle Albumin-Bound (nab-Paclitaxel) is a formulation that improves drug delivery by encapsulating paclitaxel in albumin nanoparticles, thereby enhancing tumor uptake andreducing the need for solubilizing agents like Cremophor EL, which are associated with toxicity (166,167).
Paclitaxel is associated with several adverse effects, which can vary in severity depending on the dose and combination with other agents. Common side effects include hematological toxicity, such as neutropenia, anemia, and thrombocytopenia (172), as well as non-hematological toxicity, including fatigue, peripheral neuropathy, and infections (169).
Paclitaxel has been investigated in combination with various agents to enhance its efficacy and overcome resistance. Key combination therapies include: paclitaxel and gemcitabine combination has been explored in both first-line and second-line settings for advanced urothelial carcinoma. A phase II trial reported an
overall response rate of 40% and a median overall survival of 11.8 months (173,174). This combination is administered as follows: gemcitabine 1000 mg/m² on days 1, 8, and 15, plus paclitaxel 200 mg/m² on day 1 (173,174). In a study combining gemcitabine with paclitaxel, grade 3-4 fatigue occurred in 15% of patients (175).
Paclitaxel can also be combined with immune checkpoint inhibitors, such as pembrolizumab, tremelimumab, and durvalumab. The combination of paclitaxel and pembrolizumab has shown promising activity in platinum-refractory urothelial carcinoma, with an overall response rate of 33% and a median overall survival of 12.4 months (175,176). This combination is administered through a dose of Nab-Paclitaxel 100-125 mg/m² on days 1 and 8, plus pembrolizumab 200 mg on day 1 (175,176). In a phase II trial of nab-paclitaxel and pembrolizumab, 56% of patients reported grade 3/4neutropenia, fatigue, and anemia (176). In addition, a phase I/II study combining paclitaxel with tremelimumab (an anti-CTLA4 antibody) and durvalumab (an anti-PD-L1 antibody) demonstrated an overall response rate of 26% in heavily pretreated patients with metastatic urothelial carcinoma(176,177). This combination is administered through a dose of Paclitaxel 70 mg/m² on days 1, 8,and 15, plus tremelimumab 750 mg (177). The combination of paclitaxel with immune checkpoint inhibitors can lead to immune-related adverse events, such as pneumonitis and nephritis, though these are less common (177,178).
6.6. Docetaxel
Docetaxel is a chemotherapeutic agent used in the treatment of urothelial cancer, primarily by disrupting microtubule dynamics through its ability to stabilize them and prevent their disassembly.
This disruption of mitosis leads to cell-cycle arrest and apoptosis. It also promotes apoptosis through BCL2 phosphorylation, enhancing its antitumor activity (179).
It is administered intravenously, often in combination with other agents, and has demonstrated efficacy in various regimens. Docetaxel is administered as a 1-hour infusion every 3 weeks at doses of 75 mg/m² or 100 mg/m², depending on the specific regimen and patient condition (180,181).
Its use is associated with several adverse effects, including common adverse effects such as
neutropenia, fluid retention, and neuropathy, which can lead to treatment cessation in some cases (182).
Docetaxel has also been combined with agents like ramucirumab and icrucumab in trials, aiming to improve progression-free survival in metastatic urothelial carcinoma (183).
6.7. Cyclophosphamide
Cyclophosphamide is a chemotherapeutic agent used in the treatment of various cancers, including urothelial cancer, due to its ability to induce cell death through its alkylating properties. It functions by interfering with DNA replication, thereby preventing cell division and ultimately leading to apoptosis in cancer cells (184). At high doses, it acts as an immunosuppressive agent, whereas at lower, metronomic doses, it can stimulate the immune system to target and attack cancer cells (184).
Cyclophosphamide is typically administered intravenously, either as a bolus or as a continuous infusion. Doses vary depending on the regimen: for example, 500 mg/m² in combination therapies or300-400 mg/m² per day for continuous infusion over five days (185,186).
There are common adverse effects associated with cyclophosphamide administration, including myelosuppression, nausea, vomiting, and potential for hemorrhagic cystitis (185). Moreover, Long term use can increase the risk of secondary cancers, such as bladder cancer, necessitating regular monitoring (187).
Cyclophosphamide is often combined with other drugs, such as doxorubicin, cisplatin, and methotrexate, in regimens like CAP-M and CF-Mito, which have shown efficacy in treating advanced urothelial cancers (188,186). These combinations aim to maximize therapeutic effects while minimizing toxicity, with some regimens showing partial remission in patients (188).
The choice of regimen and combination with other drugs is crucial to balance efficacy and safety, particularly in cancers like urothelial cancer, where treatment options may be limited. Regular monitoring and dose adjustments are vital for optimizing outcomes and managing adverse effects.
Cisplatin-based regimens, such as CISCA, are used in the treatment of urothelial cancer. CISCA consists of cisplatin, cyclophosphamide, and doxorubicin. Common adverse effects of CISCA may include nausea, vomiting, myelosuppression, and nephrotoxicity, primarily due to cisplatin and cyclophosphamide. A study for treating metastatic urothelial tumors indicates that MVAC is preferable over CISCA, as it achieves a higher response rate (65% vs. 46%) and longer survival duration (mean survival of 62.6 weeks for MVAC vs. 40.4 weeks for CISCA) (189).
6.8. Gemcitabine
Gemcitabine, a nucleoside analog, has emerged as a critical chemotherapeutic agent in the treatment of various cancers, including urothelial carcinomas. Gemcitabine functions as a deoxycytidine analog, inhibiting DNA synthesis by incorporating into DNA strands and causing chain termination.
It is a prodrug that requires intracellular activation to exert its cytotoxic effects. Gemcitabine’s unique properties include efficient phosphorylation, slow elimination, and self-potentiation mechanisms, which enhance its efficacy against a wide range of tumors. These characteristics make gemcitabine particularly effective in targeting rapidly dividing cancer cells while maintaining a favorable toxicity profile (190).
Gemcitabine is primarily administered intravenously for systemic treatment of advanced or metastatic urothelial cancers. However, intravesical administration has also been explored for non muscle invasive bladder cancer (NMIBC) and may hold promise for urethral cancer treatment.
Studies have demonstrated that intravesical gemcitabine is well-tolerated and effective in BCG
exposed or BCG-naïve patients, with minimal systemic toxicity (191,192).
Thedosing of gemcitabine varies depending on the treatment regimen and route of administration. For advanced urothelial carcinoma, gemcitabine is commonly administered at doses ranging from 700 mg/m² to 1,250 mg/m² (193). For intravesical use, gemcitabine is typically administered at a dose of 2,000 mg, often in combination with other agents, such as BCG or docetaxel. For example, in a Phase II trial for BCG-naïve NMIBC, gemcitabine was given weekly for 6 weeks as induction therapy, followed by monthly maintenance (192).
Gemcitabine is generally well-tolerated, but it can cause several adverse effects, mainly when administered intravenously. Common side effects include hematologic toxicity, including myelosuppression, including neutropenia, thrombocytopenia, and anemia, as well as fatigue, nausea, and vomiting (195). Less common but serious adverse effects include febrile neutropenia, renal
toxicity, and hypersensitivity reactions (196).
6.9 .Vinblastine
Vinblastine, a vinca alkaloid, plays a significant role in the treatment of urothelial cancer, mainly when used in combination with other chemotherapeutic agents. Vinblastine exerts its antineoplastic effects by binding to tubulin, a key component of microtubules, which are essential for cell division.
This binding inhibits the polymerization of tubulin into microtubules, leading to cell cycle arrest at the metaphase and ultimately inducing apoptosis in rapidly dividing cancer cells. This mechanism is well-documented in various cancers, including urothelial malignancies (201).
Vinblastine is typically administered intravenously (IV). This route ensures rapid distribution and optimal bioavailability. In some cases, particularly for localized tumors, intra-arterial administration has been explored, though it is associated with higher local toxicity compared to IV delivery (198).
Thedosing of vinblastine varies depending on the treatment regimen and the type of cancer being treated. In the context of urothelial cancers, standard dosages include 0.7 mg/m²/day. This is the recommended starting dose for prolonged continuous IV infusion, as determined in Phase I and pharmacological studies (199). Vinblastine can also be used in a dose of 2.5 mg/m² every 2 weeks: This dose was used in combination with piroxicam in a study on invasive urothelial carcinoma, demonstrating enhanced remission rates (200).
Vinblastine is associated with several adverse effects, primarily due to its impact on rapidly dividing normal cells. Common toxicities include: Myelosuppression: Leukopenia and thrombocytopenia, Peripheral Neuropathy, are dose-limiting toxicities, particularly with prolonged infusions (197).
6.10. Methotrexate
Methotrexate is a chemotherapeutic agent that has been widely used in the treatment of various cancers, including urothelial cancer. Methotrexate is a structural analogue of folic acid and functions as an antifolate drug. It inhibits dihydrofolate reductase (DHFR), an enzyme essential for the synthesis of tetrahydrofolate, which is required for DNA synthesis and cell replication. By blocking DHFR, methotrexate disrupts the synthesis of DNA and RNA, leading to cell cycle arrest and apoptosis in rapidly dividing cancer cells (201,202). In the context of urethral cancer, methotrexate’s ability to target rapidly proliferating cancer cells makes it an effective component of chemotherapy regimens. Its mechanism of action is further enhanced when combined with other chemotherapeutic agents, as it can potentiate the effects of these drugs (203).
Methotrexate can be administered through various routes, including intravenous (IV), intramuscular (IM), and oral routes. However, in the treatment of urothrlial cancer, the intravenous route is most commonly used due to its higher bioavailability and faster onset of action (202,204). In some cases, methotrexate is also administered via a hepatic arterial infusion or as part of a regional chemotherapy regimen, particularly for localized or advanced tumors. This approach enables the delivery of higher concentrations of the drug directly to the tumor site, thereby minimizing systemic toxicity (205).
The dosage of methotrexate in the treatment of urothelial cancer varies depending on the specific regimen and the patient’s overall health. Typical doses range from 30 mg/m² to 50 mg/m², administered every 1 to 4 weeks. Higher doses, such as 1000 mg/m², have been explored in clinical trials but are associated with increased toxicity and require leucovorin rescue to mitigate adverse effects (206).
Methotrexate is associated with several adverse effects, which can range from mild to severe. Common side effects include myelosuppression, which can cause neutropenia, anemia, and thrombocytopenia (207,208). Elevated liver enzymes and hepatic dysfunction have been observed (202,205). Renal Toxicity (207), Gastrointestinal Toxicity: Nausea, vomiting, and mucositis, especially with prolonged use (208).
6.11. Vinblastine and Methotrexate combination regimen
Vinblastine and methotrexate is rarely used as a monotherapy in urethral cancer treatment. Instead, it is often combined with other chemotherapeutic agents to enhance efficacy. Below are some notable combination regimens.
6.11.1 M-VECA (Methotrexate, Vinblastine, Epirubicin, Carboplatin)
This combination achieves a 50% overall response rate, with a median response duration of 50 weeks. It is less toxic than regimens containing cisplatin. However, this combination is associated with common adverse effects such as leukothrombocytopenia and mucositis (209).
6.11.2 CAMV(Carboplatin, Methotrexate, Vinblastine)
This regimen is active in advanced urothelial cancer, with a 33.4% partial response rate, a median survival of 12.5 months and a 6% complete response rate. It is particularly effective in patients with adequate renal function. This combination is generally well-tolerated, with mild myelosuppression and gastrointestinal disturbances (210).
6.11.3 CMV(Cisplatin, Methotrexate, and Vinblastine)
This regimen has been shown to improve survival rates compared to methotrexate and vinblastine alone, with a hazard ratio of 0.68 in favor of CMV (211).
This regimen is a standard treatment for advanced urothelial cancers and is associated with a median survival of 12-14 months (212,217). The MVAC regimen has demonstrated superior response rates and longer survival durations compared to the CISCA regimen in patients with metastatic urothelial tumors, achieving a combined complete and partial response rate of 65% versus 46% for CISCA, and a longer median survival duration (213). The MVAC regimen is associated with significantt oxicity, including higher rates of neutropenia, neutropenic fever, and mucositis compared to GC (214). However, accelerated MVAC (AMVAC) has been reported to be well-tolerated with manageable toxicities, making it a viable option for minimizing delays to definitive treatment (215).
The use of granulocyte colony-stimulating factor (G-CSF) is more frequent in patients receiving MVACdue to its higher toxicity profile, which includes severe neutropenia (216).
6.12. Gemcitabine regimen combination
Gemcitabine is often used in combination with other chemotherapeutic agents or targeted therapies to enhance efficacy. Key combination regimens include:
6.12.1. Gemcitabine and Cisplatin (GC)
This combination is a standard first-line regimen for advanced metastatic urothelial carcinoma (218,220). In a study of 55 patients, the GC regimen achieved an objective response rate of 63.6%, with a median overall survival of 12 months. In this combination,gemcitabine is administered at a dose of 1,000 mg/m² on days 1, 8, and 15, and cisplatin is administered at a dose of 70 mg/m² on day 1, repeated every 28 days (218). In a Phase II trial of gemcitabine and cisplatin, grade 3-4 neutropenia and thrombocytopenia were observed in 32.7% and 43.6% of patients, respectively (218).
Additionally, non-hematological effects, such as nausea and vomiting, were reported (219). GC is associated with fewer severe side effects compared to the MVAC regimen, particularly in terms of neutropenia and mucositis (219). GC is generally preferred due to a better safety profile, with fewer severe side effects and lower rates of febrile neutropenia compared to MVAC. Both GC and MVAC show similar overall survival rates in patients with urothelial carcinoma (220,221,222).
6.12.2. Gemcitabine and Nab-Paclitaxel (GA)
In a Phase III trial, the GA combination demonstrated similar efficacy to GCb, with a mediancprogression-free survival of 6.7 months. The GA regimen also showed better tolerability, with fewer grade 3-4 thrombocytopenias (223). In this combination, Gemcitabine is administered at 1,000 mg/m² on days 1 and 8, and nab-paclitaxel is administered at 125 mg/m² on days 1 and 8 (every 21 days) (223).
6.12.3. Immunotherapy Combinations
Emerging data suggest that gemcitabine can be combined with immune checkpoint inhibitors, such as pembrolizumab or tislelizumab, to enhance antitumor activity. For example, a Phase Ib/II trial of pembrolizumab in combination with gemcitabine or docetaxel demonstrated tolerability in patients who had previously received platinum treatment (224).
6.13. Carboplatin-based regimens:
Carboplatin-based therapy is a key alternative for treating urothelial cancer in patients who are ineligible for cisplatin. Cisplatin is often combined with other chemotherapies for use in various settings, including non-muscle-invasive and metastatic urothelial cancers, with a median overall survival of approximately9.3 months (225,226).
Carboplatin is typically administered with 1000 mg/m² gemcitabine on days 1 and 8, and carboplatin with an area under the curve of 4.5 every 21 days (229). A Phase II trial reported an objective response rate of 38.4%, with manageable toxicity as only 11% of patients experienced febrile neutropenia (229). This combination is frequently used due to its more tolerable adverse effects profile compared with cisplatin based therapies, which are recommended as a first-line treatment by the National Comprehensive Cancer Network (225,226,227).
Despite that, Many patients require dose reductions due to side effects, and starting with a reduced dose may minimize treatment interruptions (226).
Other combinations include methotrexate/carboplatin/vinblastine (M-CAVI), although gemcitabine/carboplatin (GC) tends to have a better overall response rate and lower severe toxicity (227,228).
Although there are common adverse effects, including thrombocytopenia, renal toxicity, and neutropenic fever, the incidence of severe acute toxicities is generally lower with GC compared to M-CAVI(227,228).
Hence, the GC combination is preferable in patients with kidney dysfunction or poor performance status (227,228).Asignificant number of patients require hospitalization for adverse event management, although this is less frequent with GC compared to other regimens (229).

Avelumab, an immunotherapy agent, has been shown to significantly prolong overall survival in patients with advanced urothelial carcinoma who have not progressed after first-line platinum-based chemotherapy. Studies indicate that avelumab maintenance therapy, when combined with best supportive care, extends survival without significantly compromising quality of life (230,231,232). The JAVELIN Bladder 100 study demonstrated that patients receiving avelumab lived longer compared to those receiving only supportive care, with a notable survival benefit observed even two years post-treatment initiation(232,233).
Another immunotherapy, pembrolizumab, has been evaluated as a maintenance therapy. It has shown to prolong progression-free survival in patients with metastatic urothelial cancer who achieved stable disease with first-line chemotherapy. This approach, known as “switch maintenance,” leverages the benefits of PD 1 blockade to delay disease progression (233).
Inaphase 2 trial, vinflunine maintenance therapy was compared to best supportive care in patients with advanced urothelial carcinoma. The study found that vinflunine extended progression-free survival compared to supportive care alone, suggesting its potential as a maintenance option, although it was associated with higher rates of adverse events(234).
Maintenance therapy, particularly with immunotherapy agents like avelumab and pembrolizumab, offers promising benefits in prolonging survival for patients with advanced urothelial carcinoma.
However, the management of urethral cancer remains complex, necessitating personalized treatment strategies and further research to enhance therapeutic outcomes.
(1) https://www.cancer.org/cancer/types/bladder-cancer/about/what-is-bladder-cancer.html#:~:text=Urothelial%20carcinoma%2C%20also%20known%20as,to%20be%20a%20urothelial%20carcinoma.
(2) https://my.clevelandclinic.org/health/diseases/6239-transitional-cell-cancer
(3) Alghafees, Mohammad A et al. “Bladder Cancer in Saudi Arabia: A Registry-Based Nationwide Descriptive Epidemiological and Survival Analysis.” Annals of Saudi Medicine 42.1 (2022): 17–28.
Web.
(4) Burger, Maximilian, et al. “Epidemiology and risk factors of urothelial bladder cancer.” European urology 63.2 (2013): 234-241.
(5) Alharbi, Hulayel, et al. “Saudi Oncology Society and Saudi Urology Association combined clinical management guidelines for urothelial cell carcinoma of the urinary bladder 2017.” Urology Annals 10.2
(2018): 133-137.
6. [Mayo Clinic – Bladder Cancer: Symptoms and Causes](https://www.mayoclinic.org/diseases-conditions/bladder-cancer/symptoms-causes/syc20356104)
7. Yaya, Manal, and Abeer H. A. Amer. “Urinary Bladder Cancer – Epidemiological and Histopathological Study.” *International Journal of Multidisciplinary Research and Analysis* 6.3 (2023): n. pag. Web.
8. Al Saidi, Ibrahim Khalid et al. “Epidemiology of Bladder Cancer in the Arab World: 2019 Global Burden of Disease Data.” *Asian Pacific Journal of Cancer Prevention* 23.9 (2022): 2907–2919. Web.
9. Abomelha, Mohammed S. “Genito-Urinary Cancer in Saudi Arabia.” *Saudi Medical Journal* 25.5 (2004): 552–556. Web.
10. Shadab, Rangrez et al. “Risk Factors for Bladder Cancer: Results of a Survey of Hospital Patients.” *Journal of Cancer and Allied Specialties* 9.1 (2022): n. pag. Web.
[https://journals.sfu.ca/jcas/index.php/jcas/article/download/485/473](https://journals.sfu.ca/jcas/index.php/jcas/article/download/485/473)
11. Letasiova, S., Medveďová, A., Sovcikova, A., Dusinska, M., Volkovova, K., Mosoiu, C., & Bartonova, A. “Bladder Cancer: A Review of the Environmental Risk Factors.” *Environmental Health* 11.S1 (2012): S11. [https://doi.org/10.1186/1476-069X-11-S1-S11](https://doi.org/10.1186/1476-069X-11-S1-S11)
12. “Smoking is the most well-established risk factor for bladder cancer in Saudi Arabia. The prevalence of smoking among Saudis is high, with 45.3% of males and 11.11% of females reporting current smoking habits.”
13. Dalela, D. “Cigarette Smoking and Urinary Bladder Cancer: The Danger Alarm Is Screaming!” *Journal of Environmental Biology* (2024): n. pag. Web.
14. Al-Shakour, Abdulkader Abdulwahab, Lamia M. Al-Naama, and Narjis A.H. Ajeel. “Smoking and Urinary Bladder Cancer: A Case-Control Study in Basrah.” *Medical Journal of Basrah University* 32.1 (2014): 1–7. Web.
15. Adly, Heba M., and Saleh A. K. Saleh. “The Association of Increased Oxidative Stress and Tumor Biomarkers Related to Polyaromatic Hydrocarbons Exposure for Different Occupational Workers in Makkah, Saudi Arabia.” *Cureus* 14 (2022): n. pag. Web.
16. Goonewardene, Sanchia S. et al. “Epidemiology, Risk Factors and Occupational Hazards.” Springer, Cham, 2021. 13–21. Web.
17. Elhawary, Nasser A. et al. “Combined Genetic Biomarkers Confer Susceptibility to Risk of Urothelial Bladder Carcinoma in a Saudi Population.” *Disease Markers* 2017 (2017): 1474560. Web.
18. van Rhijn, B. W. G., van der Kwast, T. H., Vis, A. N., Kirkels, W. J., Boevé, E. R., Jöbsis, A. C., & Zwarthoff, E. C. “FGFR3 and P53 Characterize Alternative Genetic Pathways in the Pathogenesis of Urothelial Cell Carcinoma.” *Cancer Research* 64.6 (2004): 1911–1914. [https://doi.org/10.1158/0008-5472.CAN-03-2421](https://doi.org/10.1158/0008-5472.CAN-03-2421)
19. Gonzalez, Nicole et al. “Nitrated Polycyclic Aromatic Hydrocarbon (Nitro-PAH) Signatures and Somatic Mutations in Diesel Exhaust-Exposed Bladder Tumors.” *Cancer Epidemiology, Biomarkers & Prevention* (2023): n. pag. Web.
20. Gonzalez, Nicole et al. “Data from Nitrated Polycyclic Aromatic Hydrocarbon (Nitro-PAH) Signatures and Somatic Mutations in Diesel Exhaust-Exposed Bladder Tumors.” (2023): n. pag. Web.
(21) Shamseddine, Ali et al. “Genomic Profiling of Bladder Cancer in Waterpipe and Cigarette Smokers: Implications for Carcinogenicity and Genetic Landscape.” Journal of Clinical Oncology
(2024): n. pag. Web.
(22) Koutros, Stella et al. “Targeted Deep Sequencing of Bladder Tumors Reveals Novel Associations between Cancer Gene Mutations and Mutational Signatures with Major Risk
Factors.” Clinical Cancer Research 27.13 (2021): 3725–3733. Web.
(23) Brait, Mariana et al. “Genome-Wide Methylation Profiling and the PI3K-AKT Pathway Analysis Associated with Smoking in Urothelial Cell Carcinoma.” Cell Cycle 12.7 (2013): 1058–1070. Web.
(24) Sjödahl, Gottfrid et al. “A Systematic Study of Gene Mutations in Urothelial Carcinoma; Inactivating Mutations in TSC2 and PIK3R1.” PLOS ONE 6.4 (2011): n. pag. Web.
(25) Chen, Meng et al. “Genetic Variations in PI3K-AKT-mTOR Pathway and Bladder Cancer Risk.” Carcinogenesis 30.12 (2009): 2047–2052. Web.
(26) Ouerhani, Slah, and Amel Benammar Elgaaied. “The Mutational Spectrum of HRAS, KRAS, NRAS FGFR3 Genes in Bladder Cancer.” Criminal Behaviour and Mental Health 10.6 (2012): 259–266.
Web.
(27) Mitra, Anirban P. et al. “Risk Factors and Molecular Features Associated with Bladder Cancer Development.” Springer, Cham, 2018. 3–28. Web.
(28) Xu, Ting et al. “Comprehensive FGFR3 Alteration-Related Transcriptomic Characterization Is Involved in Immune Infiltration and Correlated with Prognosis and Immunotherapy Response of Bladder Cancer.” Frontiers in Immunology 13 (2022): n. pag. Web.
(29) Martignoni, Guido. “Discovering Smoking-Related Pathway Alterations in Urothelial Cell Carcinoma Pathogenesis.” Cell Cycle 12.10 (2013): 1483. Web.
(30) Brait, Mariana et al. “Genome-Wide Methylation Profiling and the PI3K-AKT Pathway Analysis Associated with Smoking in Urothelial Cell Carcinoma.” Cell Cycle 12.7 (2013): 1058–1070. Web.
(31) Hupe, Marie C., Thomas R. Herrmann, and Axel S. Merseburger. “Upper Urinary Tract Cancer.” Springer Berlin Heidelberg, 2014. 295–298. Web.
(32) Lizardi-Calvaresi, Anne E., Staci Mitchell, and Julie Derossett. “Bladder and Urothelial Cancer.” Springer, Cham, 2020. 361–382. Web.
(33) Genega, Elizabeth M., and Christopher R. Porter. “Urothelial Neoplasms of the Kidney and Ureter. An Epidemiologic, Pathologic, and Clinical Review.” American Journal of Clinical
Pathology 117 (2002): 36. Web.
(34) Kattan, Said, et al. “The clinicopathological features of bladder carcinoma among Saudis in Riyadh Central Hospital.” Annals of Saudi Medicine 14.2 (1994): 114-116.
(35) P. Naga Jyothi et al. “Carcinoma of bladder: A rare case report.” World Journal of Biology Pharmacy and Health Sciences (2023). https://doi.org/10.30574/wjbphs.2023.13.1.0017.
(36) Alexander de J. Rafaelano M. et al. “Urothelial bladder carcinoma with major clinical presentation as overactive bladder, without hematuria: case report and literature review.” International Journal of Reproduction, Contraception, Obstetrics and Gynecology (2018). https://doi.org/10.18203/23201770.IJRCOG20184170.
(37) A. Agrawal et al. “Pulmonary manifestations of urothelial carcinoma of the bladder..” Respiratory medicine, 128 (2017): 65-69 . https://doi.org/10.1016/j.rmed.2017.05.006.
(38) G. Taarnhøj et al. “Patient-Reported Outcomes, Health-Related Quality of Life, and Clinical Outcomes for Urothelial Cancer Patients Receiving Chemo- or Immunotherapy: A Real-Life Experience.” Journal of Clinical Medicine, 10 (2021). https://doi.org/10.3390/jcm10091852.
(39) Matthew I. Milowsky et al. “Patient-Reported Outcomes in Patients With Advanced Urothelial
Cancer Who Are Ineligible for Cisplatin and Treated With First-Line Enfortumab Vedotin Alone or With Pembrolizumab.” Journal of Clinical Oncology, 42 (2024): 1403 – 1414.
https://doi.org/10.1200/JCO.23.01547.
(40) Sarafina Urenna Otis et al. “The association between paraneoplastic neurological syndromes (PNS) and urothelial carcinoma- A review of the literature..” Critical reviews in oncology/hematology (2024): 104314 . https://doi.org/10.1016/j.critrevonc.2024.104314.
(41) https://my.clevelandclinic.org/health/diseases/6239-transitional-cell-cancer#symptoms-and causes
(44) “Urothelial Carcinoma (Bladder Cancer and Upper Tracts).” Elsevier eBooks, 2023. 309-329.e4. Web.
(42) M. Rouprêt et al. “European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2017 Update..” European urology, 73 1 (2015): 111-122 . https://doi.org/10.1016/j.eururo.2017.07.036.
(43) M. Rouprêt et al. “European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update..” European urology, 59 4 (2011): 584-94 . https://doi.org/10.1016/j.eururo.2010.12.042.
(44) https://my.clevelandclinic.org/health/diseases/6239-transitional-cell-cancer#symptoms-and-causes
(45) Hemalatha, J et al. “A Clinicopathological Study of Neoplastic Urinary Bladder Lesions with Special Emphasis on
the Role of Urine Sediment Cytology in Their Diagnoses.” 2.1 (2019): 228–235. Web.
(46) Miyake, Makito et al. “Emerging Biomarkers for the Diagnosis and Monitoring of Urothelial Carcinoma.” Research and Reports in Urology 10 (2018): 251–261. Web.
(47) Kravchuk, Anton P. et al. “Urine-Based Biomarker Test Uromonitor® in the Detection and Disease Monitoring of
Non-Muscle-Invasive Bladder Cancer—A Systematic Review and Meta-Analysis of Diagnostic Test Performance.” Cancers (2024): n. pag. Web.
(48) Wu, Junlong et al. “Clinical Effectiveness of a Multitarget Urine DNA Test for Urothelial Carcinoma Detection: A Double-Blinded, Multicenter, Prospective Trial.” Molecular Cancer 23 (2024): n. pag. Web.
(49) Tsikitas, Lucas A. et al. “Imaging in Upper Tract Urothelial Carcinoma: A Review.” Cancers (2023): n. pag. Web.
(50) Laukhtina, Ekaterina, Dina Muin, and S. Shariat. “Imaging for Upper Tract Urothelial Carcinoma: Update of the Evidence and a Glimpse into the Future.” Current Opinion in Urology (2024): n. pag. Web.
(51) Patschan, O. et al. “Diagnostik von Urothelkarzinomen Des Oberen Harntrakts.” Urologe A 47.11 (2008): 1487 1496. Web.
(52) Ladetsky, Lynn, Harold A. Mitty, and Rosaleen B. Parsons. “Diagnostic Imaging in Urothelial Cancer.” (2001): 85 102. Web.
(53) M. Hong et al. “Biomarkers for Precision Urothelial Carcinoma Diagnosis: Current Approaches and the Application of Single-Cell Technologies.” Cancers, 13 (2021). https://doi.org/10.3390/cancers13020260.
(54) M. Burger et al. “ICUD-EAU International Consultation on Bladder Cancer 2012: Non-muscle-invasive urothelial carcinoma of the bladder..” European urology, 63 1 (2013): 36-44 . https://doi.org/10.1016/j.eururo.2012.08.061.
(55) Karl, Alexander et al. “Diagnostik Des Harnblasenkarzinoms.” Urologe A 47.3 (2008): 357–367. Web.
(56) Kriegmair, M. C., Ritter, M., Michel, M. S., & Bolenz, C. (2017). Modern endoscopic imaging tools for urothelial carcinoma of the urinary bladder. Aktuelle Urologie, 48(4), 296–305. https://doi.org/10.1055/S-0043-109820
(57) Mainali, Nirajan et al. “Spectrum of Urothelial Lesions in Cystoscopic Biopsies: A Histopathological Perspective.” Journal of Nobel Medical College 7.1 (2018): 6–10. Web.
(58) Ahmad, Nishat, Saurabh Banerjee, and A K. Srivastava. “Histopathological Spectrum of Urothelial Lesions: An
Observational Study.” Paripex Indian Journal Of Research (2020): 1–4. Web.
(59) E. Compérat et al. “Clinicopathological characteristics of urothelial bladder cancer in patients less than 40 years old.”
Virchows Archiv, 466 (2015): 589-594. https://doi.org/10.1007/s00428-015-1739-2.
(60) Woo, S., & Cho, J. Y. (2018). Bladder Cancer: Imaging (pp. 87–122). Academic Press. https://doi.org/10.1016/B978-0-12
809939-1.00008-4
(61) Arshia, Asma et al. “Urinary Tract Cytology Showing Variant Morphology and Divergent Differentiation.” Cytopathology (2023): n. pag. Web.
(62) Park, Jeong Hwan, and Kyung Chul Moon. “Tumor, Nodes, Metastases (TNM) Classification System for Bladder Cancer.” Academic Press, 2018. 181–184. Web.
(63) Rizzo, Alessandro et al. “TNM Staging towards a Personalized Approach in Metastatic Urothelial Carcinoma: What Will the
Future Be like?—A Narrative Review.” Translational Andrology and Urology 10.3 (2021): 1541–1552. Web.
(64) Witjes, J. Alfred et al. “European Association of Urology Guidelines on Muscle-Invasive and Metastatic Bladder Cancer:
Summary of the 2023 Guidelines.” European Urology (2023): n. pag. Web.
(65) Chromecki, Thomas F. et al. “Prognostic Factors for Upper Urinary Tract Urothelial Carcinoma.” Nature Reviews
Urology 8.8 (2011): 440–447. Web.
(66) Duquesne, Igor et al. “Lymphadenectomy for Upper Tract Urothelial Carcinoma: A Systematic Review.” Journal of Clinical
Medicine 8.8 (2019): 1190. Web.
(67) Sanjana Ranganathan et al. “Chemotherapy, immunotherapy, or combination first-line
treatment for metastatic urothelial carcinoma of the bladder: A large real-world experience..”
Journal of Clinical Oncology (2023). https://doi.org/10.1200/jco.2023.41.6_suppl.477.
(68) J. Leow et al. “A contemporary review of management and prognostic factors of upper
tract urothelial carcinoma..” Cancer treatment reviews, 41 4 (2015): 310-9 .
https://doi.org/10.1016/j.ctrv.2015.02.006.
(69) M. Abufaraj et al. “Micropapillary Urothelial Carcinoma of the Bladder: A Systematic
Review and Meta-analysis of Disease Characteristics and Treatment Outcomes..” European
urology, 75 4 (2019): 649-658 . https://doi.org/10.1016/j.eururo.2018.11.052.
(70) R. Cathomas et al. “The 2021 Updated European Association of Urology Guidelines on
Metastatic Urothelial Carcinoma..” European urology (2021).
https://doi.org/10.1016/j.eururo.2021.09.026.
(83) Berkha Rani et al. “Current and Emerging Strategies to Treat Urothelial Carcinoma.”
Cancers, 15 (2023). https://doi.org/10.3390/cancers15194886.
(84) V. Koshkin et al. “Therapy Attrition and Lessons for Future Approaches to Treatment of
Advanced Urothelial Cancer..” JAMA network open, 7 5 (2024): e249426 .
https://doi.org/10.1001/jamanetworkopen.2024.9426.
(85) Zainfeld, Daniel, and Siamak Daneshmand. “Transurethral Resection of Bladder Tumors:
Improving Quality Through New Techniques and Technologies.” Current Urology Reports 18.5 (2017):
34. Web.
(86) Ouzaid, Idir et al. “Contemporary Surgical and Technical Aspects of Transurethral Resection of
Bladder Tumor.” Translational Andrology and Urology 8.1 (2019): 21–24. Web.
(87) Jahnen, Matthias et al. “Transurethral Resection of the Urinary Bladder : Status Quo and Outlook
on New Developments.” Urologe A 60.11 (2021): 1416–1423. Web.
(88) Pearce, Shane M., and Siamak Daneshmand. “Enhanced Endoscopy in Bladder Cancer.” Current
Urology Reports 19.10 (2018): 84. Web.
(89) Chang, Timothy et al. “Image-Guided Transurethral Resection of Bladder Tumors – Current
Practice and Future Outlooks.” 3.3 (2017): 149–159. Web.
(90) Połom, Wojciech, Karol Polom, and Marcin Matuszewski. “Near Infrared Fluorescence
Applications in Urinary Bladder Cancer.” Springer, Cham, 2020. 203–211. Web.
(91) Fu, Weijun, and Xu Zhang. “Laparoscopic and Robotic-Assisted Extended Pelvic Lymph Node
Dissection for Invasive Bladder Cancer: A Review.” Bladder 10 (2023): e21200004. Web.
(92) Bloom, Jonathan, and John Phillips. “Surgical Advances in Nephroureterectomy: Laparoscopic
and Robotic Approaches.” Springer, Cham, 2018. 185–200. Web.
(93) Gallioli, Andrea et al. “New Technologies for Nephron-Sparing Surgery in Upper Urinary Tract
Cancers.” Current Opinion in Urology 33 (2023): 510–515. Web.
(94) Shao, I-Hung et al. “Recent Advances in Upper Tract Urothelial Carcinomas: From Bench to
Clinics.” International Journal of Urology 26.2 (2019): 148–159. Web.
(95) Pavlov, V., M. F. Urmantsev, and M. R. Bakeev. “Robot-Assistant Radical Cystectomy as a
Modern Method of Personalized Treatment for Patients with Muscle-Invasive Bladder Cancer.”
(2024): n. pag. Web.
(96) Pavlov, Valentin, M. F. Urmantsev, and M. R. Bakeev. “The Success of Robot-Assisted
Cystectomy in the Treatment of Muscle-Invasive Bladder Cancer.” Onkourologiâ 18.2 (2022): 123
128. Web.
(97) O’Rourke, Timothy K., Parth U. Thakker, and Ashok K. Hemal. “Refined Step-by-Step Narrative
Review of Robotic Radical Nephroureterectomy in the Management of Upper Tract Urothelial
Carcinoma.” Translational Andrology and Urology (2023): n. pag. Web.
(98) Guru, Khurshid A. et al. “A Robotic Future for Bladder Cancer.” Lancet Oncology 9.2 (2008): 184.
Web.
(99) Liu, Ming-Zhu et al. “Radiation Therapy for Nonmetastatic Medically Inoperable Upper-Tract
Urothelial Carcinoma.” Translational Andrology and Urology 10.7 (2021): 2929–2937. Web.
(100) Khriguian, Julia et al. “Stereotactic Ablative Radiation Therapy for the Treatment of Upper
Urinary Tract Urothelial Carcinoma.” Practical radiation oncology (2021): n. pag. Web.
(101) Svedman, Fernanda Costa et al. “Stereotactic Body Radiation Therapy Is Beneficial for a
Subgroup of Patients with Urothelial Cancer and Solitary Metastatic Disease: A Single Institution
Real-World Experience.” Radiation Oncology 19.1 (2024): n. pag. Web.
(102) Francolini, Giulio et al. “Stereotactic Radiotherapy in Oligoprogressive and Oligorecurrent
Urothelial Cancer Patients: A Retrospective Experience.” Cancer treatment and research 19
(2019): 100124. Web.
(103) Vassiliou, Vassilios et al. “Radiotherapy in Metastatic Urothelial Carcinoma: Rationale and
Clinical Applications.” Anticancer Research 42.8 (2022): 3767–3778. Web.
(104) Peshin, S., S. Modi, and S. Singh. “Advancements in Cancer Immunotherapy: A Comprehensive
Review of Immune Checkpoint Inhibitors with a Focus on Pembrolizumab and Emerging Strategies.”
Medi Clin Case Rep J 2.3 (2024): 430-434.
(105) Baldawi, Muaamar B., et al. “Adverse endocrine-related effects of pembrolizumab precipitating
severe hyponatremia.” Cureus 14.8 (2022).
(106) Banerjee, Abha, Chandrakala Aluganti Narasimhulu, and Dinender K. Singla. “Immune
interactions in pembrolizumab (PD-1 inhibitor) cancer therapy and cardiovascular complications.”
American Journal of Physiology-Heart and Circulatory Physiology 325.4 (2023): H751-H767.
(107) Bajorin, Dean F. et al. “KEYNOTE-052: Phase 2 study of pembrolizumab (MK-3475) as first-line
therapy for patients (pts) with unresectable or metastatic urothelial cancer ineligible for cisplatin
based therapy.” J ournal of Clinical Oncology 33 (2015): n. pag.
(108) Balar, Arjun V. et al. “Pembrolizumab as first-line therapy in cisplatin-ineligible advanced
urothelial cancer: Results from the total KEYNOTE-052 study population.” J ournal of C linical
Oncology 35 (2017): 284-284.
(109) Ashfaq, Ammar, Nishanth Thalambedu, and Muhammad Umair Atiq. “Acute pancreatitis
secondary to pembrolizumab-induced hypertriglyceridemia.” Cureus 15.4 (2023).
(110) Hsu, Fu-Shun, Chun-Hung Su, and Kou-How Huang. “A Comprehensive Review of US FDA
Approved Immune Checkpoint Inhibitors in Urothelial Carcinoma.” Clinical & Developmental
Immunology 2017.2017 (2017): 6940546. Web.
(111) Alves, Ana Caroline Fonseca et al. “Immunotherapy plus Chemotherapy versus Chemotherapy
Alone as First-Line Treatment for Advanced Urothelial Cancer: A Systematic Review and Meta
Analysis of Randomized Controlled Trials.” Journal of Clinical Oncology 42.16_suppl (2024): e16554.
Web.
(112) Califano, Gianluigi et al. “Immune Checkpoint Inhibition in Upper Tract Urothelial Carcinoma.”
World Journal of Urology 39.5 (2021): 1357–1367. Web.
(113) Patel, Aakash et al. “Immune Checkpoint Inhibitors in the Management of Urothelial Carcinoma.” J
Cancer Immunol (Wilmington) 3.2 (2021): 115–136. Web.
(114) Kato, Minoru, and Junji Uchida. “Recent Advances in Immune Checkpoint Inhibitors in the
Treatment of Urothelial Carcinoma: A Review.” International Journal of Urology 30 (2023): n. pag. Web.
(115) Erdafitinib (Balversa) for Urothelial Carcinoma. (2024). Deleted Journal, 66(1702), e83–e84.
https://doi.org/10.58347/tml.2024.1702g
(116) Loriot, Y. et al. “Erdafitinib or Chemotherapy in Advanced or Metastatic Urothelial Carcinoma.”
The New England Journal of Medicine (2023): n. pag. Web.
(117) Rouvinov, Keren et al. “Erdafitinib Treatment in Metastatic Urothelial Carcinoma: A Real-World
Analysis.” Frontiers in Oncology 13 (2023): n. pag. Web.
(118) Siefker-Radtke, A. et al. “Erdafitinib versus Pembrolizumab in Pretreated Patients with Advanced
or Metastatic Urothelial Cancer with Select FGFR Alterations: Cohort 2 of the Randomized Phase III
THOR Trial.” Annals of Oncology (2023): n. pag. Web.
(119) Vlachou, Evangelia, Burles A. Johnson, and Jean H. Hoffman-Censits. “The Role of Antibody-Drug
Conjugates in Urothelial Cancer: A Review of Recent Advances in the Treatment of Locally Advanced and
Metastatic Urothelial Cancer.” Clinical Medicine: Oncology 18 (2024): n. pag. Web.
(120) Ruder, S., Martínez, J., Palmer, J., Arham, A., & Tagawa, S. T. (2025). Antibody–drug conjugates in
urothelial carcinoma: current status and future. Current Opinion in Urology.
https://doi.org/10.1097/mou.0000000000001263
(121) Renee Hein et al. “Abstract 1901: Disitamab vedotin, a clinical stage HER2-directed antibody-drug
conjugate, shows potent antitumor activity as a monotherapy in preclinical urothelial cancer
models.” Cancer Research (2024). https://doi.org/10.1158/1538-7445.am2024-1901.
(122) Jing Jiang et al. “Preclinical safety profile of Disitamab vedotin:a novel anti-HER2 antibody
conjugated with MMAE..” Toxicology letters (2019). https://doi.org/10.1016/j.toxlet.2019.12.027.
(123) Chenglong Li et al. “Efficacy and safety of disitamab vedotin combined with toripalimab as
neoadjuvant treatment in patients with HER2 positive locally advanced muscle-invasive urothelial bladder
cancer..” Journal of Clinical Oncology (2024). https://doi.org/10.1200/jco.2024.42.4_suppl.631.
(124) Zhenyu Ou et al. “Efficacy and safety of neoadjuvant therapy with disitamab vedotin plus
toripalimab in treating muscle invasive urothelial bladder cancer followed by selective bladder-preserving
or radical cystectomy..” Journal of Clinical Oncology (2024).
https://doi.org/10.1200/jco.2024.42.16_suppl.e16590.
(125) Taylor, Caroline et al. “Mechanistic Insights into the Successful Development of Combination
Therapy of Enfortumab Vedotin and Pembrolizumab for the Treatment of Locally Advanced or Metastatic
Urothelial Cancer.” Cancers 16 (2024): n. pag. Web.
(126) You, Xiangyun et al. “Emerging Strategy for the Treatment of Urothelial Carcinoma: Advances in
Antibody-Drug Conjugates Combination Therapy.” 171 (2024): 116152. Web.
(127) Benjamin, David J., and Robert Hsu. “Treatment Approaches for FGFR-Altered Urothelial Carcinoma:
Targeted Therapies and Immunotherapy.” Frontiers in Immunology 14 (2023): n. pag. Web.
(128) Hsu, Fu-Shun, Chun-Hung Su, and Kou-How Huang. “A Comprehensive Review of US FDA
Approved Immune Checkpoint Inhibitors in Urothelial Carcinoma.” Clinical & Developmental Immunology
2017.2017 (2017): 6940546. Web.
(129) Shepherd, Jonathan et al. “Prediction of Immune Checkpoint Inhibitor (ICI) Response in Patients with
Urothelial Cancer (UC) by a Novel RNA-Based ICI Response Signature (ICI-PRS).” Journal of Clinical
Oncology 42.16_suppl (2024): e16502. Web.
(130) Xie, Deqian et al. “The Mechanism and Clinical Application of DNA Damage Repair Inhibitors
Combined with Immune Checkpoint Inhibitors in the Treatment of Urologic Cancer.” Frontiers in Cell and
Developmental Biology 11 (2023): n. pag. Web.
(131) Grisay, Guillaume et al. “Future Strategies Involving Immune Checkpoint Inhibitors in Advanced
Urothelial Carcinoma.” Current Treatment Options in Oncology 22.1 (2021): 1–24. Web.
(132) Fei Li et al. “Regulation of cisplatin resistance in bladder cancer by epigenetic
mechanisms..” Drug resistance updates : reviews and commentaries in antimicrobial and anticancer
chemotherapy, 68 (2023): 100938 . https://doi.org/10.1016/j.drup.2023.100938.
(133) Andrea M P Romani et al. “Cisplatin in Cancer Treatment..” Biochemical pharmacology (2022):
115323 . https://doi.org/10.1016/j.bcp.2022.115323.
(134) Shaloam Dasari et al. “Cisplatin in cancer therapy: molecular mechanisms of action..” European
journal of pharmacology, 740 (2014): 364-78 . https://doi.org/10.1016/j.ejphar.2014.07.025.
(135) R. O’Dwyer et al. “Split-Dose Cisplatin in Patients With Locally Advanced or Metastatic Urothelial
Carcinoma: A Systematic Literature Review and Network Meta-Analysis..” Clinical genitourinary cancer,
22 6 (2024): 102176 . https://doi.org/10.1016/j.clgc.2024.102176.
(136) R. O’Dwyer et al. “Clinical outcomes with split-dose cisplatin-based regimens in patients (pts) with
locally advanced or metastatic urothelial carcinoma (la/mUC): Results of a systematic literature review
(SLR) and network meta-analysis (NMA)..” Journal of Clinical Oncology (2024).
https://doi.org/10.1200/jco.2024.42.4_suppl.589.
(137) G. Iyer et al. “Urological Oncology: Bladder, Penis and Urethral Cancer, and Basic Principles of
Oncology Re: Multicenter Prospective Phase II Trial of Neoadjuvant Dose-Dense Gemcitabine plus
Cisplatin in Patients with Muscle-Invasive Bladder Cancer.” Journal of Urology (2020).
https://doi.org/10.1097/JU.0000000000000729.
(138) J. Coen et al. “Bladder Preservation With Twice-a-Day Radiation Plus Fluorouracil/Cisplatin or
Once Daily Radiation Plus Gemcitabine for Muscle-Invasive Bladder Cancer: NRG/RTOG 0712-A
Randomized Phase II Trial..” Journal of clinical oncology : official journal of the American Society of
Clinical Oncology, 37 1 (2019): 44-51 . https://doi.org/10.1200/JCO.18.00537.
(139) R. Ali et al. “Can Cisplatin Therapy Be Improved? Pathways That Can Be Targeted.” International
Journal of Molecular Sciences, 23 (2022). https://doi.org/10.3390/ijms23137241.
(140) Lazzeri, Massimo et al. “Randomized Phase III Clinical Trial of Neoadjuvant Intravesical
Mitomycin C (MMC) Treatment in Patients with Primary Treatment-Naïve Non-Muscle Invasive
Bladder Cancer (NMIBC).” Journal of Clinical Oncology 41.6_suppl (2023): TPS578. Web.
(141) Pourmadadi, Mehrab et al. “Macromolecules and Nanomaterials Loaded with Mitomycin C as
Promising New Treatment Option Cancer Drug Nanoformulation: A Literature Review.” Journal of
Drug Delivery Science and Technology (2023): n. pag. Web.
(142) Mashi, Sharfuddeen Abbas et al. “Low Grade Transitional Cell Carcinoma of the Urethra
Successfully Treated with Only Intraurethral Instillation of Mitomycin-C.” 1.2 (2018): n. pag. Web.
(143) Melonakos, Emmanuel J., and Richard A. Santucci. “Treatment of Low-Grade Bulbar
Transitional Cell Carcinoma with Urethral Instillation of Mitomycin C.” Advances in Urology
2008.2008 (2008): 173694. Web.
(144) Draeger, D., and O. W. Hakenberg. “Feasibility and Effectiveness of Second-Line
Chemotherapy with Mitomycin C in Patients with Advanced Penile Cancer.” Frontiers in urology
(2024): n. pag. Web.
(145) Ali, Liaqat et al. “Efficacy of Mitomycin C in Reducing Recurrence of Anterior Urethral Stricture
after Internal Optical Urethrotomy.” Korean Journal of Urology 56.9 (2015): 650–655. Web.
(146) Al-Falah, A.H. et al. “Efficacy of Mitomycin c in Reducing the Recurrence of Urethral Stricture
after Visual Internal Urethrotomy (VIU).” 5.1 (2020): 289–291. Web.
(147) Prakash, Jvs et al. “Intravesical Mitomycin c versus Gemcitabine Instillation in the Treatment of
Low-Risk Non-Muscle Invasive Bladder Cancer: An Analytical Study.” Indian journal of applied
research (2023): n. pag. Web.
(148) Alghorabi, Amal A., Ahmed M. Kabel, and Maaly A. Abd Elmaaboud. “Doxorubicin: Insights into
Dynamics, Clinical Uses and Adverse Effects.” The Journal of Cancer Research 7.1 (2019): 17–20.
Web.
(149) Tacar, Oktay, Pornsak Sriamornsak, and Crispin R. Dass. “Doxorubicin: An Update on Anticancer
Molecular Action, Toxicity and Novel Drug Delivery Systems.” Journal of Pharmacy and Pharmacology
65.2 (2012): 157–170. Web.
(150) Kciuk, Mateusz et al. “Doxorubicin—An Agent with Multiple Mechanisms of Anticancer Activity.”
Cells 12.4 (2023): 659. Web.
(151) Kang, S. H., McDermott, C., Farr, S., & Chess-Williams, R. (2015). Enhanced urothelial ATP
release and contraction following intravesical treatment with the cytotoxic drug, doxorubicin. Naunyn
Schmiedebergs Archives of Pharmacology, 388(7), 773–780. https://doi.org/10.1007/S00210-015
1097-2
(152) See, William A. et al. “Continuous Infusion, Intravesical Doxorubicin for the Treatment of
Regionally Advanced Bladder Cancer: A Phase I-II Trial.” American Journal of Clinical Oncology 20.4
(1997): 331–337. Web.
(153) Alghorabi, Amal A., Ahmed M. Kabel, and Maaly A. Abd Elmaaboud. “Doxorubicin: Insights into
Dynamics, Clinical Uses and Adverse Effects.” The Journal of Cancer Research 7.1 (2019): 17–20.
Web.
(154) Luo, Yang et al. “The Apoptosis Mechanism of Epirubicin Combined with BCG on Human
Bladder Cancer Cells.” Anti-cancer Agents in Medicinal Chemistry 20.13 (2020): 1571–1581. Web.
(155) Niijima, Tadao et al. “Cooperative Phase II Study of Epirubicin (EPI) in Bladder Cancer, Renal
Pelvic and Ureteral Tumors–Urological Cooperative Study Group of EPI.” Hinyokika kiyo. Acta
urologica Japonica 32.9 (1986): 1359. Web.
(156) Tran, Thi Hien et al. “Long‐term Prevention of Bladder Cancer Progression by Alpha1‐oleate
Alone or in Combination with Chemotherapy.” International Journal of Cancer 153.3 (2023): 584
599. Web.
(157) Onrust, Susan V., Lynda R. Wiseman, and Karen L. Goa. “Epirubicin: A Review of Its
Intravesical Use in Superficial Bladder Cancer.” Drugs & Aging 15.4 (1999): 307–333. Web.
(158) Shang, Pan Feng et al. “Intravesical Bacillus Calmette-Guérin versus Epirubicin for Ta and T1
Bladder Cancer.” Cochrane Database of Systematic Reviews 5 (2011): n. pag. Web.
(159) Lu, Jia-wei et al. “Transurethral Holmium Laser Resection and Transurethral Electrocision
Combined with Intravesical Epirubicin within 24 Hours Postoperatively for Treatment of Bladder
Cancer.” Journal of International Medical Research 48.6 (2020): 300060519887267. Web.
(160) Rozzi, Antonio et al. “Weekly Regimen of Epirubicin and Paclitaxel as Second-Line Chemotherapy in
Patients with Metastatic Transitional Cell Carcinoma of Urothelial Tract: Results of a Phase II Study.”
Medical Oncology 28.1 (2011): 426–432. Web.
(161) Lelli, G. et al. “Chemotherapy with Cisplatin, Epirubicin, Methotrexate in the Treatment of Locally
Advanced or Metastatic Transitional Cell Cancer of the Bladder (TCC).” Journal of Chemotherapy 4.4
(1992): 239–243. Web.
(162) Uekado, Yasunari et al. “The Effects of Intravesical Chemoimmunotherapy with Epirubicin and
Bacillus Calmette-Guérin for Prophylaxis of Recurrence of Superficial Bladder Cancer: A Preliminary
Report.” Cancer Chemotherapy and Pharmacology 35.1 (1994): n. pag. Web.
(163) Raitanen, M P, and Olavi Lukkarinen. “A Controlled Study of Intravesical Epirubicin with or without
Alpha2b‐interferon as Prophylaxis for Recurrent Superficial Transitional Cell Carcinoma of the Bladder.”
BJUI 76.6 (1995): 697–701. Web.
(164) Neri, Bruno et al. “Gemcitabine plus Epi-Doxorubicin as First-Line Chemotherapy for Bladder Cancer
in Advanced or Metastatic Stage: A Phase II.” Anticancer Research 22.5 (2002): 2981–2984. Web.
(165) Ohuchi, Hideki et al. “Study of Neoadjuvant Chemotherapy for Invasive Bladder Cancer with MEC
(Methotrexate, Epirubicin, Cisplatin) Therapy.” Hinyokika kiyo. Acta urologica Japonica 47.4 (2001): 241
246. Web.
(166) Abaza, Yasmin, and Carlos Alemany. “Nanoparticle Albumin-Bound-Paclitaxel in the
Treatment of Metastatic Urethral Adenocarcinoma: The Significance of Molecular Profiling and
Targeted Therapy.” Case reports in urology 2014 (2014): 489686. Web.
(167) Sharifi-Rad, Javad et al. “Paclitaxel: Application in Modern Oncology and Nanomedicine
Based Cancer Therapy.” Oxidative Medicine and Cellular Longevity 2021 (2021): 3687700. Web.
(168) Bokemeyer, Carsten et al. “Recent Strategies for the Use of Paclitaxel in the Treatment of
Urological Malignancies.” World Journal of Urology 16.2 (1998): 155–162. Web.
(169) Lee, Jae-Lyun et al. “Phase II Study of a Cremophor-Free, Polymeric Micelle Formulation of
Paclitaxel for Patients with Advanced Urothelial Cancer Previously Treated with Gemcitabine and
Platinum.” Investigational New Drugs 30.5 (2012): 1984–1990. Web.
(170) Liu, Xiangrui et al. “Pharmacokinetics, Tissue Distribution and Anti-Tumour Efficacy of
Paclitaxel Delivered by Polyvinylpyrrolidone Solid Dispersion.” Journal of Pharmacy and
Pharmacology 64.6 (2012): 775–782. Web.
(171) Tamura, Koetsu et al. “Therapeutic Effect of Intravesical Administration of Paclitaxel
Solubilized with Poly(2-Methacryloyloxyethyl Phosphorylcholine-Co-n-Butyl Methacrylate) in an
Orthotopic Bladder Cancer Model.” BMC Cancer 15.1 (2015): 317. Web.
(172) Alva, Ajjai et al. “Phase II Trial of Combination Nab-Paclitaxel, Carboplatin and Gemcitabine in First
Line Therapy of Advanced Urothelial Carcinoma.” Investigational New Drugs 32.1 (2014): 188–194.
Web.
(173) Kanai, Kunimitsu et al. “Gemcitabine and Paclitaxel Chemotherapy for Advanced Urothelial
Carcinoma in Patients Who Have Received Prior Cisplatin-Based Chemotherapy.” International Journal of
Clinical Oncology 13.6 (2008): 510–514. Web.
(174) Kaufman, Donald S. et al. “A Multi-Institutional Phase II Trial of Gemcitabine plus Paclitaxel in
Patients with Locally Advanced or Metastatic Urothelial Cancer.” Urologic Oncology-seminars and
Original Investigations 22.5 (2004): 393–397. Web.
(175) Bitting, Rhonda L et al. “Low Dose Paclitaxel with Pembrolizumab Enhances Clinical and
Immunologic Responses in Platinum Refractory Urothelial Carcinoma.” Cancer research communications
(2024): n. pag. Web.
(176) “A Phase 2 Trial of Nab-Paclitaxel in Combination With Anti-PD1 Therapy in Advanced Urothelial
Cancer.” 209.1 (2023): 121–130. Web.
(177) Einerhand, S. et al. “LBA103 ICRA: Efficacy of Paclitaxel with Tremelimumab +/- Durvalumab in
Metastatic Urothelial Carcinoma after Progression on Platinum Chemotherapy and Anti-PD-(L)1.” Annals
of Oncology (2023): n. pag. Web.
(178) Bitting, Rhonda L et al. “Low Dose Paclitaxel with Pembrolizumab Enhances Clinical and
Immunologic Responses in Platinum Refractory Urothelial Carcinoma.” Cancer research communications
(2024): n. pag. Web.
(179) Mackler, Niklas J., and Kenneth J. Pienta. “Drug Insight: Use of Docetaxel in Prostate and
Urothelial Cancers.” Nature Clinical Practice Urology 2.2 (2005): 92–100. Web.
(180) de Wit, R. et al. “Docetaxel (Taxotere): An Active Agent in Metastatic Urothelial Cancer;
Results of a Phase II Study in Non-Chemotherapy-Pretreated Patients.” British Journal of
Cancer 78.10 (1998): 1342–1345. Web.
(181) Petrylak, Daniel P. et al. “Docetaxel As Monotherapy or Combined With Ramucirumab or
Icrucumab in Second-Line Treatment for Locally Advanced or Metastatic Urothelial Carcinoma:
An Open-Label, Three-Arm, Randomized Controlled Phase II Trial.” Journal of Clinical Oncology
34.13 (2016): 1500–1509. Web.
(182) de Wit, R. et al. “Docetaxel (Taxotere): An Active Agent in Metastatic Urothelial Cancer;
Results of a Phase II Study in Non-Chemotherapy-Pretreated Patients.” British Journal of
Cancer 78.10 (1998): 1342–1345. Web.
(183) Petrylak, Daniel P. et al. “Docetaxel As Monotherapy or Combined With Ramucirumab or
Icrucumab in Second-Line Treatment for Locally Advanced or Metastatic Urothelial Carcinoma:
An Open-Label, Three-Arm, Randomized Controlled Phase II Trial.” Journal of Clinical Oncology
34.13 (2016): 1500–1509. Web.
(184) Delahousse, J, Luiz Alberto Molina, and Angelo Paci. “Cyclophosphamide and Analogues; a Matter
of Dose and Schedule for Dual Anticancer Activities.” Cancer Letters (2024): 217119. Web.
(185) Tchekmedyian, N.S. et al. “Phase I Clinical and Pharmacokinetic Study of Cyclophosphamide
Administered by Five-Day Continuous Intravenous Infusion.” Cancer Chemotherapy and Pharmacology
18.1 (1986): 33–38. Web.
(186) Citrin, D. L. et al. “A Study of Cyclophosphamide, Adriamycin, Cis-Platinum, and Methotrexate in
Advanced Transitional Cell Carcinoma of the Urinary Tract.” Cancer 51.1 (1983): 1–4. Web.
(187) Lupera, H. et al. “Cancer de La Vessie Après Traitement Par Cyclophosphamide: À Propos d’un
Cas et Revue de La Littérature.” Journal d’urologie 96.1 (1990): 48–52. Web.
(188) Sahashi, Masafumi et al. “Combination Chemotherapy for Advanced Urothelial-Tract Carcinoma.”
Cancer Chemotherapy and Pharmacology 30.1 (1992): n. pag. Web.
(189) Logothetis, C., Dexeus, F., Finn, L., Sella, A., Amato, R., Ayala, A., & Kilbourn, R. (1990). A prospective
randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial
tumors.. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 8 6, 1050-5 .
https://doi.org/10.1200/JCO.1990.8.6.1050.
(190) Gesto, Diana S. et al. “Gemcitabine: A Critical Nucleoside for Cancer Therapy.” Current
Medicinal Chemistry 19.7 (2012): 1076–1087. Web.
(191) Gaffney, Christopher et al. “A Phase II Trial of Intravesical Chemoimmunotherapy with
Gemcitabine and Bacillus Calmette-Guérin (BCG) for Patients with BCG-Exposed, High-Grade,
Non-Muscle–Invasive Bladder Cancer.” Journal of Clinical Oncology 41.6_suppl (2023): TPS579.
Web.
(192) Patel, Sunil H. et al. “A Phase II Trial of Intravesical Gemcitabine and Docetaxel in the
Treatment of Bacillus Calmette-Guérin‒Naïve Nonmuscle-Invasive Urothelial Carcinoma of the
Bladder.” The Journal of Urology (2024): 101097JU0000000000003977. Web.
(193) Dreicer, Robert et al. “Phase 2 Trial of Pemetrexed Disodium and Gemcitabine in Advanced
Urothelial Cancer (E4802): A Trial of the Eastern Cooperative Oncology Group.” Cancer 112.12
(2008): 2671–2675. Web.
(194) Ozawa, Akira et al. “Gemcitabine and Cisplatin for Advanced Urothelial Carcinomas: The
Ehime University Hospital Experience.” International Journal of Clinical Oncology 12.4 (2007):
279–283. Web.
(195) Ikeda, Masaomi et al. “Combination of Gemcitabine and Paclitaxel Is a Favorable Option for
Patients with Advanced or Metastatic Urothelial Carcinoma Previously Treated with Cisplatin
Based Chemotherapy.” Japanese Journal of Clinical Oncology 41.10 (2011): 1214–1220. Web.
<https://academic.oup.com/jjco/article/41/10/1214/1183351>.
(196) Bamias, Aristotle et al. “The Combination of Gemcitabine and Carboplatin as First-Line
Treatment in Patients with Advanced Urothelial Carcinoma. A Phase II Study of the Hellenic
Cooperative Oncology Group.” Cancer 106.2 (2006): 297–303. Web.
(197) Ratain, Mark J., and Nicholas J. Vogelzang. “Phase I and Pharmacological Study of
Vinblastine by Prolonged Continuous Infusion.” Cancer Research 46.9 (1986): 4827–4830.
Web.
(198) Uchida, J et al. “Comparison of Side Effects Caused by Intra-Arterial and Intravenous
Infusion of M-VAC (Methotrexate, Vinblastine, Adriamycin and Cisplatin) for Urothelial Cancer.”
Hinyokika kiyo. Acta urologica Japonica 43.9 (1997): 637. Web.
(199) Ratain, Mark J., and Nicholas J. Vogelzang. “Phase I and Pharmacological Study of
Vinblastine by Prolonged Continuous Infusion.” Cancer Research 46.9 (1986): 4827–4830.
Web.
(200) Knapp, Deborah W. et al. “A Nonselective Cyclooxygenase Inhibitor Enhances the Activity
of Vinblastine in a Naturally-Occurring Canine Model of Invasive Urothelial Carcinoma.” 2.2
(2016): 241–250. Web. <https://content.iospress.com/articles/bladder-cancer/blc150044>.
(201) Koźmiński, Przemysław et al. “Overview of Dual-Acting Drug Methotrexate in Different
Neurological Diseases, Autoimmune Pathologies and Cancers.” International Journal of Molecular
Sciences 21.10 (2020): 3483. Web.
(202) Khan, Zulfequar Ahamad, R.C. Tripathi, and Brahmeshwar Mishra. “Methotrexate: A
Detailed Review on Drug Delivery and Clinical Aspects.” Expert Opinion on Drug Delivery 9.2
(2012): 151–169. Web.
(203) de Wit, Ronald, and Joaquim Bellmunt. “Chemotherapy in Metastatic Urothelial Cancer.”
American Journal of Cancer 1.1 (2002): 23–31. Web.
(204) Igawa, Mikio et al. “Preliminary Results of Concurrent Methotrexate, Cisplatin and Radiation
Treatment for Locally Advanced Urothelial Cancers.” International Urology and Nephrology 28.2
(1996): 189–194. Web.
(205) Oh, William K. et al. “A Phase II Trial of Methotrexate, Cisplatin, 5-Fluorouracil, and
Leucovorin in the Treatment of Invasive and Metastatic Urothelial Carcinoma.” Cancer 86.7
(1999): 1329–1334. Web. <https://www.ncbi.nlm.nih.gov/pubmed/10506721>.
(206) Dodd, Paul M. et al. “Phase II Trial of Intermediate Dose Methotrexate in Combination with
Vinblastine, Doxorubicin, and Cisplatin in Patients with Unresectable or Metastatic Transitional
Cell Carcinoma.” Cancer 85.5 (1999): 1145–1150. Web.
(207) Boccardo, Francesco et al. “Carboplatin, Methotrexate, and Vinblastine in the Treatment of
Patients with Advanced Urothelial Cancer : A Phase II Trial.” Cancer 73.7 (1994): 1932–1936.
Web.
(208) Halim, Amal, and Niveen Abo-Touk. “Methotrexate‐paclitaxel‐epirubicin‐carboplatin as
Second‐line Chemotherapy in Patients with Metastatic Transitional Cell Carcinoma of the Bladder
Pretreated with Cisplatin‐gemcitabine: A Phase II Study.” Asia-pacific Journal of Clinical Oncology
9.1 (2013): 60–65. Web.
(209) Lorusso, Vito et al. “M-VECA (Methotrexate, Vinblastine, Epidoxorubicin and Carboplatin) in the Treatment of
Advanced Urothelial Carcinoma.” Tumori 79.3 (1993): 191–194. Web.
(210) Boccardo, Francesco et al. “Carboplatin, Methotrexate, and Vinblastine in the Treatment of Patients with
Advanced Urothelial Cancer : A Phase II Trial.” Cancer 73.7 (1994): 1932–1936. Web.
(211) Mead, Graham M. et al. “A Randomized Trial Comparing Methotrexate and Vinblastine (MV) with Cisplatin,
Methotrexate and Vinblastine (CMV) in Advanced Transitional Cell Carcinoma: Results and a Report on Prognostic
Factors in a Medical Research Council Study. MRC Advanced Bladder Cancer Working Party.” British Journal of Cancer
78.8 (1998): 1067–1075. Web.
(212) Bellmunt, Joaquim et al. “Optimizing Therapeutic Strategies in Advanced Bladder Cancer: Update on
Chemotherapy and the Role of Targeted Agents.” Critical Reviews in Oncology Hematology 69.3 (2009): 211–222.
Web.
(213) C. Logothetis et al. “A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with
metastatic urothelial tumors..” Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 8 6
(1990): 1050-5 . https://doi.org/10.1200/JCO.1990.8.6.1050.
(214) H. von der Maase et al. “Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in
Advanced or Metastatic Bladder Cancer: Results of a Large, Randomized, Multinational, Multicenter, Phase III Study..” Journal
of clinical oncology : official journal of the American Society of Clinical Oncology, 41 23 (2023): 3881-3890 .
https://doi.org/10.1200/JCO.22.02763.
(215) Christopher Blick et al. “Accelerated MVAC as neoadjuvant chemotherapy for patients with muscle-invasive transitional
cell carcinoma of the bladder..” Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 29
7_suppl (2011): 235. https://doi.org/10.1200/JCO.2011.29.7_SUPPL.235.
(216) Se Young Choi et al. “MP32-04 GEMCITABINE–CISPLATIN VERSUS MVAC CHEMOTHERAPY IN UROTHELIAL
CARCINOMA: A NATIONWIDE COHORT STUDY.”The Journal of Urology, 209 (2023): e441.
https://doi.org/10.1097/JU.0000000000003265.04.
(217) Hibi, Hatsuki et al. “M-VAC (Methotrexate, Vinblastine, Doxorubicin and Cisplatin) for Poor Prognosis Patients
with Urothelial Tumors and Effect of Dose Intensity.” Hinyokika kiyo. Acta urologica Japonica 43.2 (1997): 89–96. Web.
(218) Ozawa, Akira et al. “Gemcitabine and Cisplatin for Advanced Urothelial Carcinomas: The Ehime University
Hospital Experience.” International Journal of Clinical Oncology 12.4 (2007): 279–283. Web.
(219) H. von der Maase et al. “Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in
Advanced or Metastatic Bladder Cancer: Results of a Large, Randomized, Multinational, Multicenter, Phase III Study..” Journal
of clinical oncology : official journal of the American Society of Clinical Oncology, 41 23 (2023): 3881-3890 .
https://doi.org/10.1200/JCO.22.02763.
(220) Se Young Choi et al. “MP32-04 GEMCITABINE–CISPLATIN VERSUS MVAC CHEMOTHERAPY IN
UROTHELIAL CARCINOMA: A NATIONWIDE COHORT STUDY.”The Journal of Urology, 209 (2023): e441.
https://doi.org/10.1097/JU.0000000000003265.04.
(221) Lee, Y., Kim, Y., Hong, B., Cho, Y., & Lee, J. (2021). Comparison of clinical outcomes in patients with localized or
locally advanced urothelial carcinoma treated with neoadjuvant chemotherapy involving gemcitabine–cisplatin and high dose
intensity MVAC. Journal of Cancer Research and Clinical Oncology, 147, 3421 – 3429. https://doi.org/10.1007/s00432-021
03582-x.
(222) H. von der Maase et al. “Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in
Advanced or Metastatic Bladder Cancer: Results of a Large, Randomized, Multinational, Multicenter, Phase III Study..” Journal
of clinical oncology : official journal of the American Society of Clinical Oncology, 41 23 (2023): 3881-3890 .
https://doi.org/10.1200/JCO.22.02763.
(223) An, Xin et al. “Gemcitabine/Nab-Paclitaxel versus Gemcitabine/Carboplatin for Advanced Urothelial Carcinoma.”
BJUI (2023): n. pag. Web.
(224) Parikh, Mamta et al. “Combination Checkpoint Immunotherapy and Cytotoxic Chemotherapy: Further Results
from a Phase Ib/II Trial of Pembrolizumab and Docetaxel or Gemcitabine in Patients with Advanced or Metastatic
Urothelial Cancer.” Journal of Clinical Oncology 36 (2018): 4541. Web.
(225) T. Freshwater et al. “Systematic Literature Review and Meta-Analysis of Response to First-Line
Therapies for Advanced/Metastatic Urothelial Cancer Patients Who Are Cisplatin Ineligible.” American
Journal of Clinical Oncology, 42 (2019): 802 – 809. https://doi.org/10.1097/COC.0000000000000585.
(226) Eleni Gamvroulas et al. “Evaluation of Gemcitabine and Carboplatin Dosing in Patients With Cisplatin
Ineligible Metastatic Urothelial Carcinoma..” Clinical genitourinary cancer, 23 1 (2024): 102279 .
https://doi.org/10.1016/j.clgc.2024.102279.
(227) M. D. De Santis et al. “Randomized phase II/III trial assessing gemcitabine/ carboplatin and
methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer “unfit” for cisplatin-based
chemotherapy: phase II–results of EORTC study 30986..” Journal of clinical oncology : official journal of the
American Society of Clinical Oncology, 27 33 (2009): 5634-9 . https://doi.org/10.1200/JCO.2008.21.4924.
(228) M. D. De Santis et al. “Randomized phase II/III trial assessing gemcitabine/carboplatin and
methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin
based chemotherapy: EORTC study 30986..” Journal of clinical oncology : official journal of the American
Society of Clinical Oncology, 30 2 (2012): 191-9 . https://doi.org/10.1200/JCO.2011.37.3571.
(229) Parmod Kumar et al. “Gemcitabine and carboplatin versus gemcitabine and paclitaxel chemotherapy in
cisplatin ineligible advanced-stage urothelial cancers: A prospective randomized control study..” Journal of
Clinical Oncology (2022). https://doi.org/10.1200/jco.2022.40.6_suppl.500.
(230) Grivas, P., Kopyltsov, E., Su, P., Parnis, F., Park, S., Yamamoto, Y., Fong, P., Tournigand, C., Duran, M., Bamias, A.,
Caserta, C., Chang, J., Cislo, P., Di Pietro, A., Wang, J., & Powles, T. (2022). Patient-reported Outcomes from JAVELIN
Bladder 100: Avelumab First-line Maintenance Plus Best Supportive Care Versus Best Supportive Care Alone for Advanced
Urothelial Carcinoma.. European urology. https://doi.org/10.1016/j.eururo.2022.04.016.
(231) Powles, T., Park, S., Voog, E., Caserta, C., Valderrama, B., Gurney, H., Kalofonos, H., Radulović, S., Demey, W., Ullén,
A., Loriot, Y., Sridhar, S., Tsuchiya, N., Kopyltsov, E., Sternberg, C., Bellmunt, J., Aragon-Ching, J., Petrylak, D., Laliberté, R.,
Wang, J., Huang, B., Davis, C., Fowst, C., Costa, N., Blake-Haskins, J., Di Pietro, A., & Grivas, P. (2020). Avelumab
Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma.. The New England journal of medicine.
https://doi.org/10.1056/NEJMoa2002788.
(232) Powles, T., Park, S., Gurney, H., Loriot, Y., Sridhar, S., Bellmunt, J., Di Pietro, A., & Grivas, P. (2025). Avelumab
maintenance treatment for advanced urothelial cancer: plain language summary of long-term results from the JAVELIN Bladder
100 study.. Future oncology, 1-12 . https://doi.org/10.1080/14796694.2024.2435208.
(233) Galsky, M., Mortazavi, A., Milowsky, M., George, S., Gupta, S., Fleming, M., Dang, L., Geynisman, D., Walling, R.,
Alter, R., Kassar, M., Wang, J., Gupta, S., Davis, N., Picus, J., Philips, G., Quinn, D., Haines, G., Hahn, N., Zhao, Q., Yu, M., &
Pal, S. (2020). Randomized Double-Blind Phase II Study of Maintenance Pembrolizumab Versus Placebo After First-Line
Chemotherapy in Patients With Metastatic Urothelial Cancer.. Journal of clinical oncology : official journal of the American
Society of Clinical Oncology, JCO1903091 . https://doi.org/10.1200/JCO.19.03091.
(234) García-Donas, J., Font, A., Pérez-Valderrama, B., Virizuela, J., Climent, M., Hernando-Polo, S., Arranz, J., Del Mar
Llorente, M., Laínez, N., Villa-Guzmán, J., Mellado, B., Del Alba, G., Castellano, D., Gallardo, E., Anido, U., Del Muro, G.,
Doménech, M., Puente, J., Morales-Barrera, R., Pérez-Gracia, J., & Bellmunt, J. (2017). Maintenance therapy with vinflunine
plus best supportive care versus best supportive care alone in patients with advanced urothelial carcinoma with a response after
first-line chemotherapy (MAJA; SOGUG 2011/02): a multicentre, randomised, controlled, open-label, phase 2 trial.. The Lancet.
Oncology, 18 5, 672-681a . https://doi.org/10.1016/S1470-2045(17)30242-5.
All Rights Reserved to Indigoarabia.com
Stay Connected for latest Genitourinary news
connect with us on LinkedIn for professional updates and collaborations.
All Rights Reserved to Indigoarabia.com