
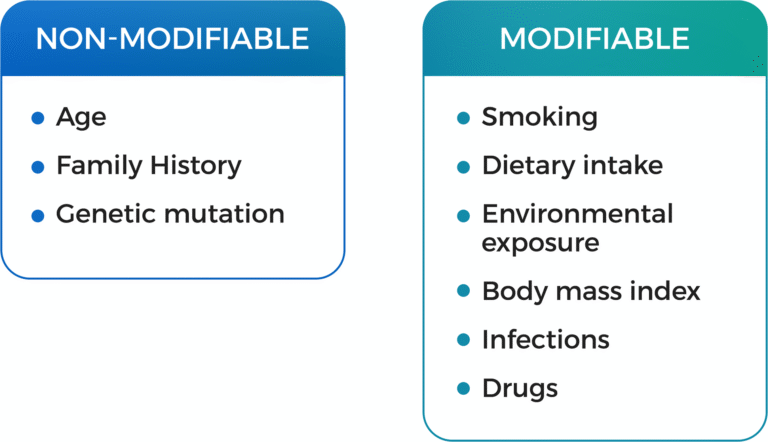
Prostate cancer stands as the second most prevalent cancer affecting males worldwide. Various risk factors contribute to its development, including familial predisposition, advancing age, lifestyle choices such as diet, and environmental influences. The onset of prostate cancer can often be traced back to cellular inflammation or genetic mutations.
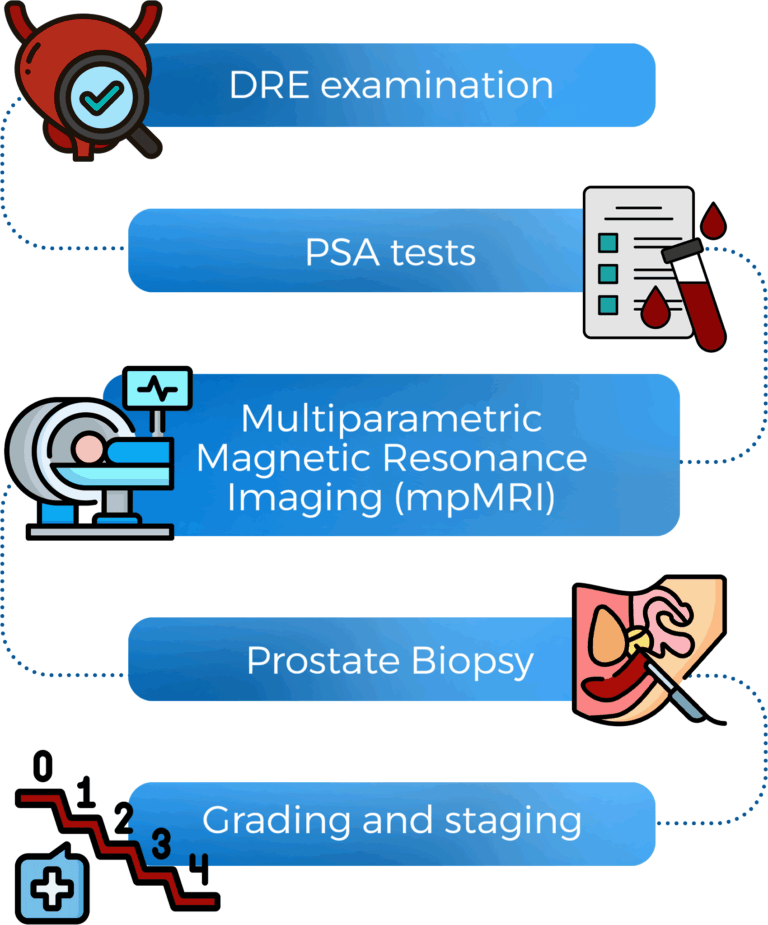
Awareness of the key symptoms is crucial for early detection. Individuals may notice signs such as blood in urine or semen, significant unintended weight loss, or discomfort during ejaculation. Diagnosis typically involves a PSA blood test or a digital rectal examination. If there is a suspicion of cancer, advanced diagnostic tools, such as MRI or biopsy, can provide essential insights into the type and stage of the disease.
The treatment landscape for prostate cancer is diverse, tailored to the specific type and stage of the cancer diagnosed. While some therapeutic approaches may carry potential side effects such as urinary or fecal incontinence and sexual dysfunction, this complexity underscores the importance of comprehensive care and informed decision-making.
In anticipation of possible sexual dysfunction, sperm banking is an invaluable option for those considering future parenthood through IVF. Furthermore, specialized treatments remain available for challenging scenarios, such as castration-resistant prostate cancer or bone metastasis.
This evolving field continuously presents new avenues of hope through targeted therapies and innovative treatments.It is essential to approach the journey of diagnosis and treatment with resilience and a proactive mindset. By educating males, seeking timely medical advice, and relying on support networks, the challenges posed by prostate cancer can be navigated, and a pathway toward recovery and well-being can be fostered. Together, through awareness and advocacy, we can inspire hope and drive progress in the fight against this disease.

Prostate cancer stands as the second most prevalent cancer affecting males worldwide. Various risk factors contribute to its development, including familial predisposition, advancing age, lifestyle choices such as diet, and environmental influences. The onset of prostate cancer can often be traced back to cellular inflammation or genetic mutations.
Awareness of the key symptoms is crucial for early detection. Individuals may notice signs such as blood in urine or semen, significant unintended weight loss, or discomfort during ejaculation. Diagnosis typically involves a PSA blood test or a digital rectal examination. If there is a suspicion of cancer, advanced diagnostic tools, such as MRI or biopsy, can provide essential insights into the type and stage of the disease.
The treatment landscape for prostate cancer is diverse, tailored to the specific type and stage of the cancer diagnosed. While some therapeutic approaches may carry potential side effects such as urinary or fecal incontinence and sexual dysfunction, this complexity underscores the importance of comprehensive care and informed decision-making.
In anticipation of possible sexual dysfunction, sperm banking is an invaluable option for those considering future parenthood through IVF. Furthermore, specialized treatments remain available for challenging scenarios, such as castration-resistant prostate cancer or bone metastasis.
This evolving field continuously presents new avenues of hope through targeted therapies and innovative treatments.It is essential to approach the journey of diagnosis and treatment with resilience and a proactive mindset. By educating males, seeking timely medical advice, and relying on support networks, the challenges posed by prostate cancer can be navigated, and a pathway toward recovery and well-being can be fostered. Together, through awareness and advocacy, we can inspire hope and drive progress in the fight against this disease.

Plant-based nutrition is associated with a lower risk of prostate cancer and a better prognosis during treatment [13].
Lycopene rich in tomatoes, is associated with reduced prostate cancer risk [14] . A high consumption of vegetables, fruits, fish, and whole grain products exerts protective and/or therapeutic effects [15] .
Phytoestrogens are found in foods such as soybeans, lentils, tofu, peanuts, chickpeas, and kidney beans in addition to specific phytoestrogens found in daidzein, genistein, and glycitein were possible protective factors against prostate cancer [16] .
While increased risk of prostate cancer is associated with a high intake of total dairy products, milk, cheese, low-fat milk, and skim milk combined, total calcium, dietary calcium, and dairy calcium, but not whole milk [17] . In addition, inflammatory and hyper-insulinemic dietary patterns such as processed meat, sugar, refined grains, and saturated fats are linked to an increased risk of prostate cancer and prostate carcinogenesis [18] since they lead to the disruption of prostate hormonal regulation, induction of oxidative stress and inflammation, and alteration of growth factor signaling and lipid metabolism [15] .
Consistent aspirin use is associated with a lower risk of the development of prostate cancer [23].
5-Alpha-reductase inhibitors, which are used for enlarged prostate and scalp hair loss, are associated with reduced prostate cancer risk [24] .
Key elements affecting risk: [6].
1.Number of affected relatives.
2.Degree of relationship (first-degree vs. second-degree).
3.Age of diagnosis and death from prostate cancer.
4.Presence of high-grade disease and history of other cancers (e.g., breast, ovarian).
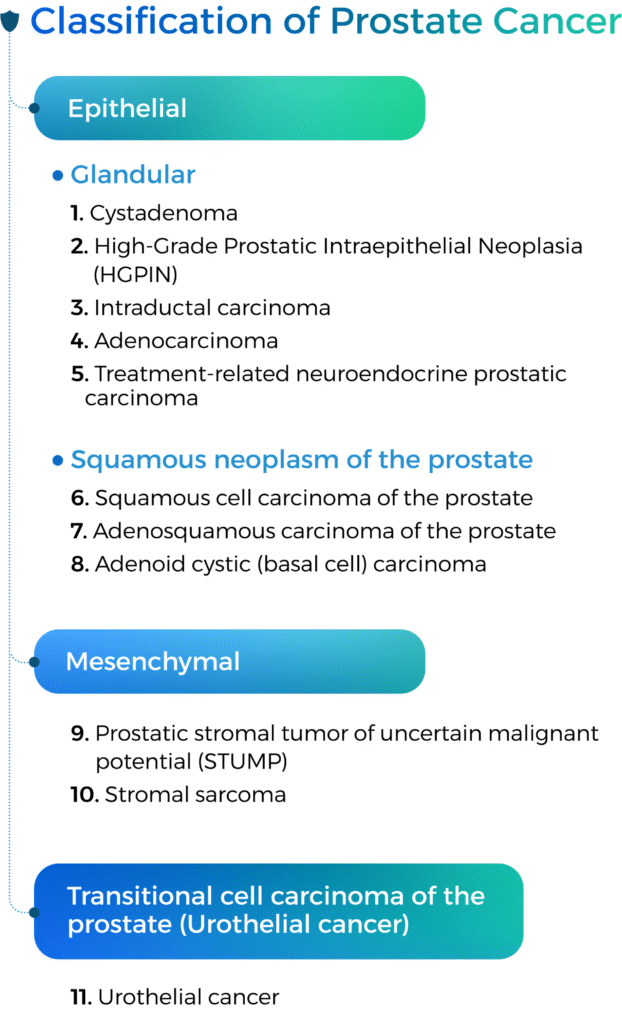
Benign tumors (ICD-O:0)
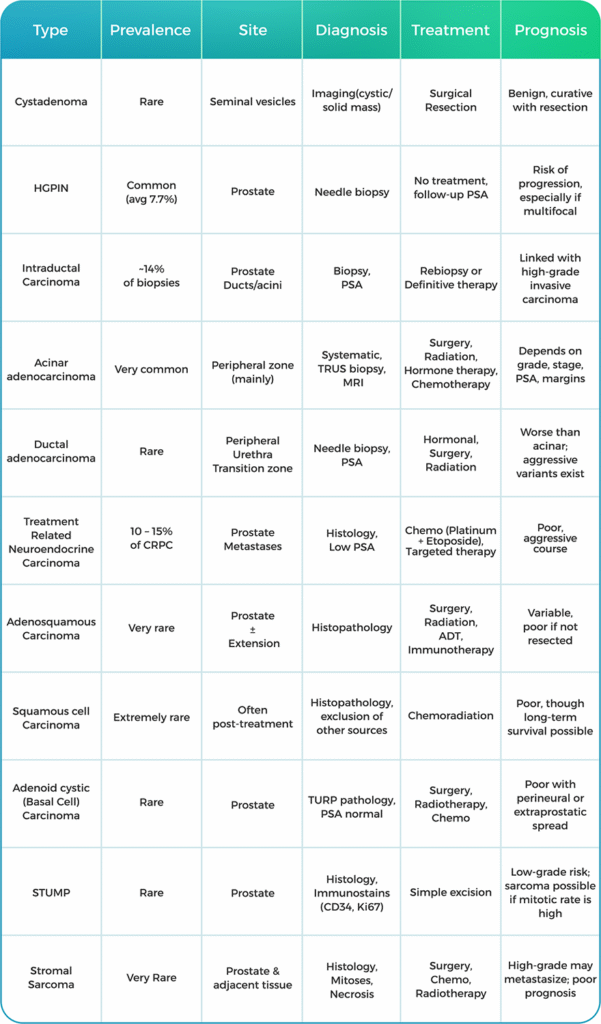
Prostatic cystadenoma is a rare, benign epithelial tumor of variably sized cysts lined by bland cuboidal cells. It is commonly centered within seminal vesicles that occur in a wide age distribution (23 66 years old).
Malignant cancer (ICD-O:3)
Benign tumors (ICD-O:0)

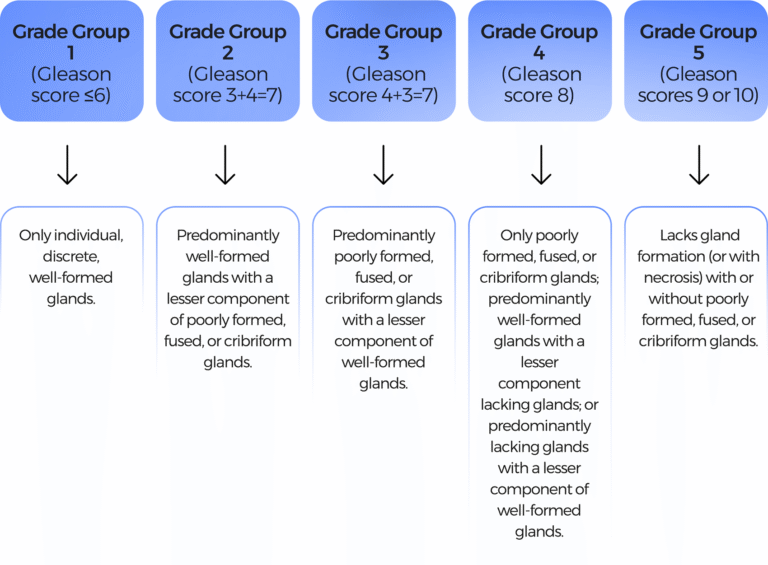
1.Gleason grading system: Grades 1-5, predicts aggressiveness
Tumor, Nodes, and Metastasis (TNM) is the system used for tumor staging. The TNM score is a measure of how far prostate cancer has spread in the body [53, 54].
The T (tumor) score rates the size and extent of the original tumor.
The N (nodes) score rates whether the cancer has spread into nearby lymph nodes.
The M (metastasis) score rates whether the cancer has spread to distant sites.
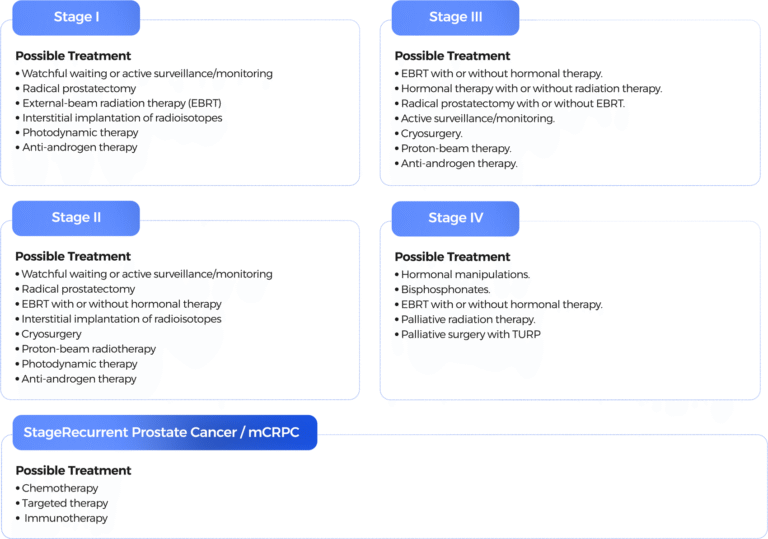
Early-stage prostate cancer remains confined to the prostate. It is also known as localized prostate cancer and may be referred to as stage I or II.
Regional prostate cancer (locally advanced prostate cancer) has extended beyond the prostate to nearby areas, such as the seminal vesicles or lymph nodes. Some forms of regional or locally advanced prostate cancer are classified as stage III.
Metastatic prostate cancer (advanced prostate cancer), or stage IV cancer, has spread (metastasized) beyond the prostate and pelvis to other parts of the body [53].
Active surveillance
Watchful waiting.
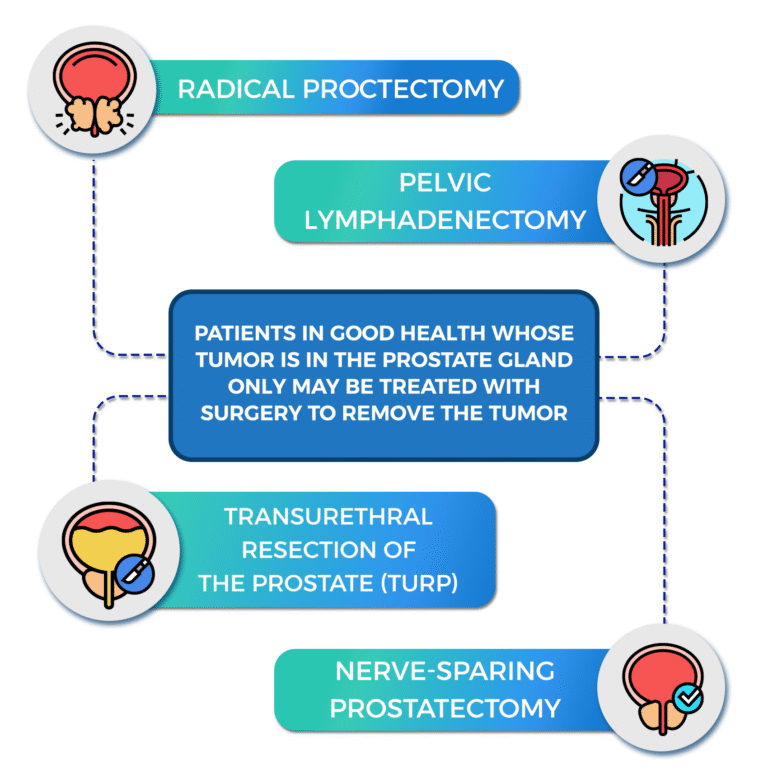
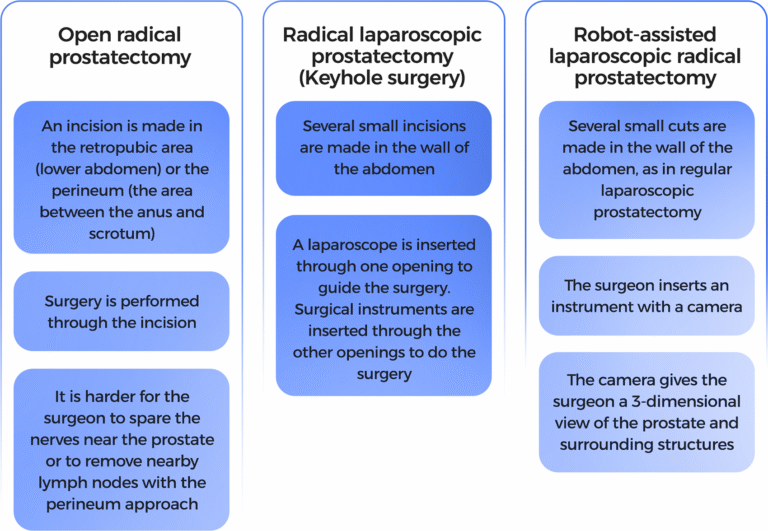
A surgical procedure to remove the prostate, surrounding tissue, and seminal vesicles. Removal of nearby lymph nodes may be done at the same time. The rate of maintenance of sexual potency with radical prostatectomy was 10% to 40%.
A surgical procedure to remove tissue from the prostate using a resectoscope inserted through the urethra.
This procedure is done to treat benign prostatic hypertrophy, and it is sometimes done to relieve symptoms caused by a tumor before other cancer treatment is given.
TURP may also be done in men whose tumor is in the prostate only and who cannot have a radical prostatectomy.
This is carried out under general anesthetic or a spinal anesthetic (epidural).
Possible problems after prostate cancer surgery include:
Using high-energy rays (like x-rays) to kill the cancer. Hormonal therapy may be used before undergoing radiotherapy to increase the chance of successful treatment and/or after radiotherapy to reduce the chances of cancerous cells recurrence.
Short-term effects of radiotherapy can include:
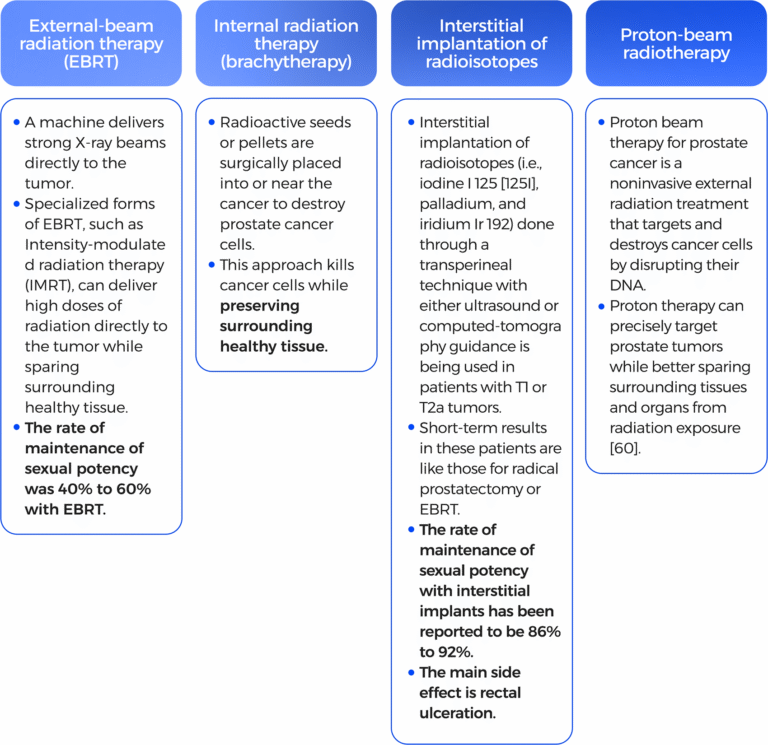
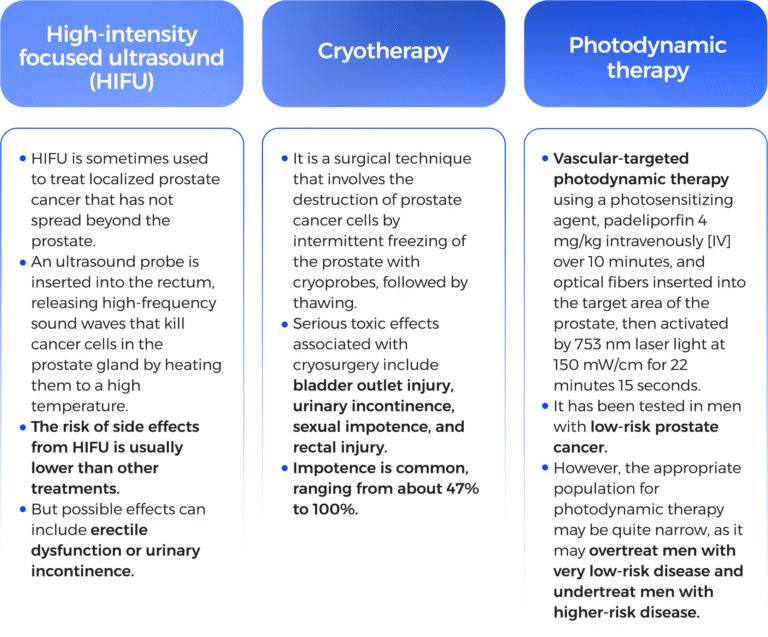
The main side effects are:
Side effects of long-term treatment are:
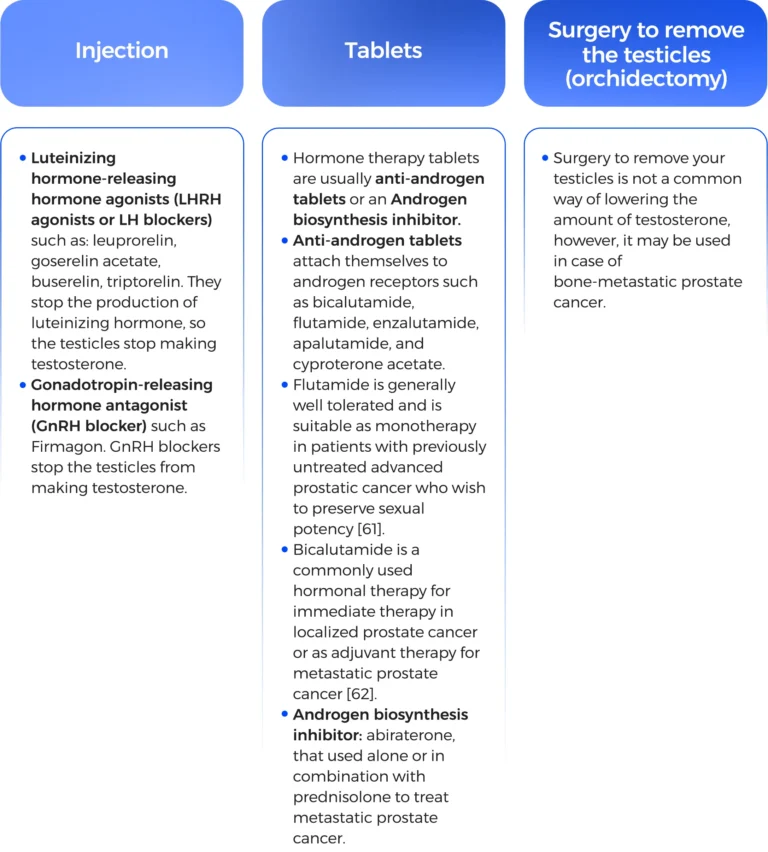
Enzalutamide significantly decreased the risk of radiographic progression and death and delayed the initiation of chemotherapy in men with metastatic prostate cancer [63].
Apalutamide exerts its action by directly inhibiting the androgen receptor at the ligand-binding domain.
This action allows for the inhibition of Androgen receptor nuclear translocation, DNA binding, and impedes AR-mediated transcription [64].
Apalutamide’s major metabolite, N-desmethyl apalutamide, is a less potent inhibitor of the androgen receptor. The inhibitory actions of apalutamide allow for decreased tumor cell proliferation and increased apoptosis, leading to decreased tumor volume. It is approved for nonmetastatic and metastatic CRPC [64].
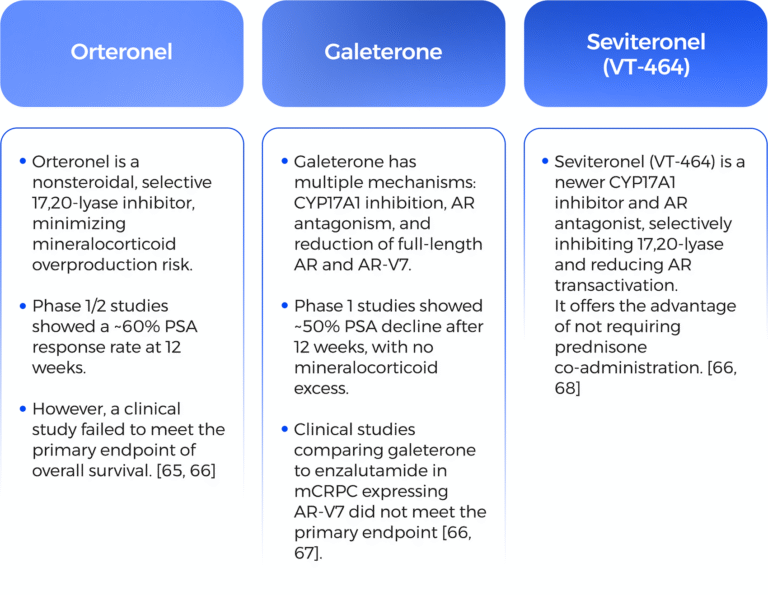


1-Abbas, N., et al., The Silent Burden of De Novo Metastatic Prostate Cancer in the Middle East: A Call for Region-Specific Screening Guidelines. Société Internationale d’Urologie Journal, 2025. 6(1): p. 4.
2-Observatory), G.G.C. 2024; Available from: https://gco.iarc.who.int/today/ https://gco.iarc.who.int/media/globocan/factsheets/cancers/27-prostate-fact-sheet.pdf.
3-Zlotta, A.R., et al., Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. Journal of the National Cancer Institute, 2013. 105(14): p. 1050-1058.
1. Abbas, N., et al., The Silent Burden of De Novo Metastatic Prostate Cancer in the Middle East: A Call for Region-Specific Screening Guidelines. Société Internationale d’Urologie Journal, 2025. 6(1): p. 4.
2. Observatory), G.G.C. 2024; Available from: https://gco.iarc.who.int/today/ https://gco.iarc.who.int/media/globocan/factsheets/cancers/27-prostate-fact-sheet.pdf.
3. Zlotta, A.R., et al., Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened Caucasian and Asian men. Journal of the National Cancer Institute, 2013. 105(14): p. 1050-1058.
4. Institute, N.C. Cancer Stat Facts: Prostate Cancer. Available from: https://seer.cancer.gov/statfacts/html/prost.html.
5. Arafa, M.A. and D.M. Rabah, With increasing trends of prostate cancer in the Saudi Arabia and Arab World: Should we start screening programs? World Journal of Clinical Oncology, 2017. 8(6): p. 447.
6. Bergengren, O., et al., 2022 Update on Prostate Cancer Epidemiology and Risk Factors—A Systematic Review. European Urology, 2023. 84(2): p. 191-206.
7. foundation, P.c. Risk factors. Available from: https://www.pcf.org/patient-resources/family-cancer-risk/prostate-cancer-risk-factors/.
8. Rajwa, P., et al., Positive family history as a predictor for disease outcomes after radical prostatectomy for nonmetastatic prostate cancer. Arab Journal of Urology, 2023. 21(4): p. 241-247.
9. Barber, L., et al., Family History of Breast or Prostate Cancer and Prostate Cancer Risk. Clin Cancer Res, 2018. 24(23): p. 5910-5917.
10. D’Elia, G., et al., Increased Risk of Hereditary Prostate Cancer in Italian Families with Hereditary Breast and Ovarian Cancer Syndrome Harboring Mutations in BRCA and in Other Susceptibility Genes. Genes (Basel), 2022. 13(10).
11. Darcey, E. and T. Boyle, Tobacco smoking and survival after a prostate cancer diagnosis: A systematic review and meta-analysis. Cancer Treatment Reviews, 2018. 70: p. 30-40.
12. Gupta, S., et al., Relationship between type of smokeless tobacco & risk of cancer: A systematic review. Indian Journal of Medical Research, 2018. 148(1).
13. Gupta, N., et al., Systematic review of the impact of a plant-based diet on prostate cancer incidence and outcomes. Prostate Cancer and Prostatic Diseases, 2022. 25(3): p. 444-452.
14. Rowles, J.L., et al., Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: a systematic review and meta-analysis. Prostate Cancer and Prostatic Diseases, 2017. 20(4): p. 361-377.
15. Oczkowski, M., et al., Dietary Factors and Prostate Cancer Development, Progression, and Reduction. Nutrients, 2021. 13(2).
16. Zhang, Q., et al., Phytoestrogens and risk of prostate cancer: an updated meta-analysis of epidemiologic studies. International journal of food sciences and nutrition, 2017. 68(1): p. 28-42.
17. Aune, D., et al., Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies23. The American Journal of Clinical Nutrition, 2015. 101(1): p. 87-117.
18. Fu, B.C., et al., Insulinemic and Inflammatory Dietary Patterns and Risk of Prostate Cancer. European Urology, 2021. 79(3): p. 405-412.
19. Prins, G.S., Endocrine disruptors and prostate cancer risk. Endocr Relat Cancer, 2008. 15(3): p. 649-56.
Saha, A., M.G. Kolonin, and J. DiGiovanni, Obesity and prostate cancer — microenvironmental roles of adipose tissue. Nature Reviews Urology, 2023. 20(10): p. 579-596.
Russo, G.I., et al., Human papillomavirus and risk of prostate cancer: a systematic review and meta-analysis. The Aging Male, 2020. 23(2): p. 132-138.
22. Hayes, R.B., et al., Sexual behaviour, STDs and risks for prostate cancer. Br J Cancer, 2000. 82(3): p. 718-25.
23. Qiao, Y., et al., Associations between aspirin use and the risk of cancers: a meta-analysis of observational studies. BMC cancer, 2018. 18: p. 1-57.
24. Hu, X., et al., Association of 5-alpha-reductase inhibitor and prostate cancer incidence and mortality: a meta-analysis. Translational andrology and urology, 2020. 9(6): p. 2519.
25. Alukal, J.P. and H. Lepor, Testosterone Deficiency and the Prostate. Urol Clin North Am, 2016. 43(2): p. 203-8.
26. Bethel, C.R., A.M. De Marzo, and W.G. Nelson, Chapter 25 – Molecular Pathogenesis of Prostate Cancer: Somatic, Epigenetic, and Genetic Alterations, in Essential Concepts in Molecular Pathology, W.B. Coleman and G.J. Tsongalis, Editors. 2010, Academic Press: San Diego. p. 335-340.
27. Leslie, S.W., T.L. Soon-Sutton, and W.P. Skelton, Prostate Cancer, in StatPearls. 2025, StatPearls Publishing
Copyright © 2025, StatPearls Publishing LLC.: Treasure Island (FL).
28. Mani, R.S., et al., Inflammation-Induced Oxidative Stress Mediates Gene Fusion Formation in Prostate Cancer. Cell Reports, 2016. 17(10): p. 2620-2631.
29. Sfanos, K.S. and A.M. De Marzo, Prostate cancer and inflammation: the evidence. Histopathology, 2012. 60(1): p. 199-215.
30. Grossmann, M., A.S. Cheung, and J.D. Zajac, Androgens and prostate cancer; pathogenesis and deprivation therapy. Best Practice & Research Clinical Endocrinology & Metabolism, 2013. 27(4): p. 603-616.
31. Azad, A.A., et al., Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clinical cancer research, 2015. 21(10): p. 2315-2324.
32. Romanel, A., et al., Plasma AR and abiraterone-resistant prostate cancer. Sci Transl Med, 2015. 7(312): p. 312re10.
33. Shorning, B.Y., et al., The PI3K-AKT-mTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling. Int J Mol Sci, 2020. 21(12).
34. Jamaspishvili, T., et al., Clinical implications of PTEN loss in prostate cancer. Nat Rev Urol, 2018. 15(4): p. 222-234.
35. Drudge-Coates, L., et al., Recognizing Symptom Burden in Advanced Prostate Cancer: A Global Patient and Caregiver Survey. Clinical Genitourinary Cancer, 2018. 16(2): p. e411-e419.
36. (CDC), C.f.D.C.a.P. Prostate cancer. Available from: https://www.cdc.gov/prostate-cancer/symptoms/index.html.
37. Netto, G.J., et al., The 2022 World Health Organization Classification of Tumors of the Urinary System and Male Genital Organs—Part B: Prostate and Urinary Tract Tumors. European Urology, 2022. 82(5): p. 469-482.
38. Zhou, M., High-grade prostatic intraepithelial neoplasia, PIN-like carcinoma, ductal carcinoma, and intraductal carcinoma of the prostate. Modern Pathology, 2018. 31(1): p. 71-79.
39. UK, C.R. Prostate cancer Available from: https://www.cancerresearchuk.org/about-cancer/prostate-cancer/stages/types
40. Pires Gama A, C.B. WHO classification of prostate cancer March 18th, 2025.]; Available from: https://www.pathologyoutlines.com/topic/prostateWHO.html.
41. Kirby, M., et al., Is the digital rectal exam any good as a prostate cancer screening test? British Journal of General Practice, 2024. 74(740): p. 137.
42. Descotes, J.-L., Diagnosis of prostate cancer. Asian Journal of Urology, 2019. 6(2): p. 129-136.
43. guidlines, N. Prostate cancer. Available from: https://www.nice.org.uk/guidance/ng131/chapter/Recommendations#assessment-and-diagnosis.
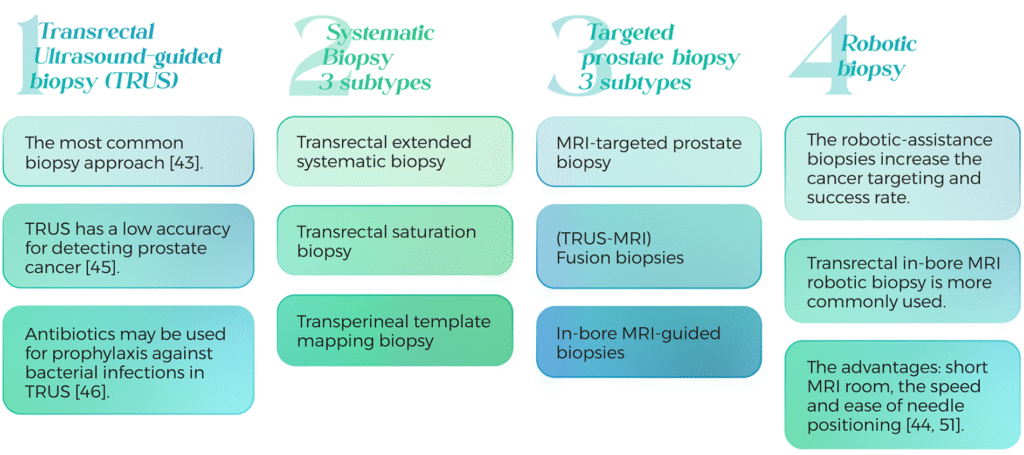
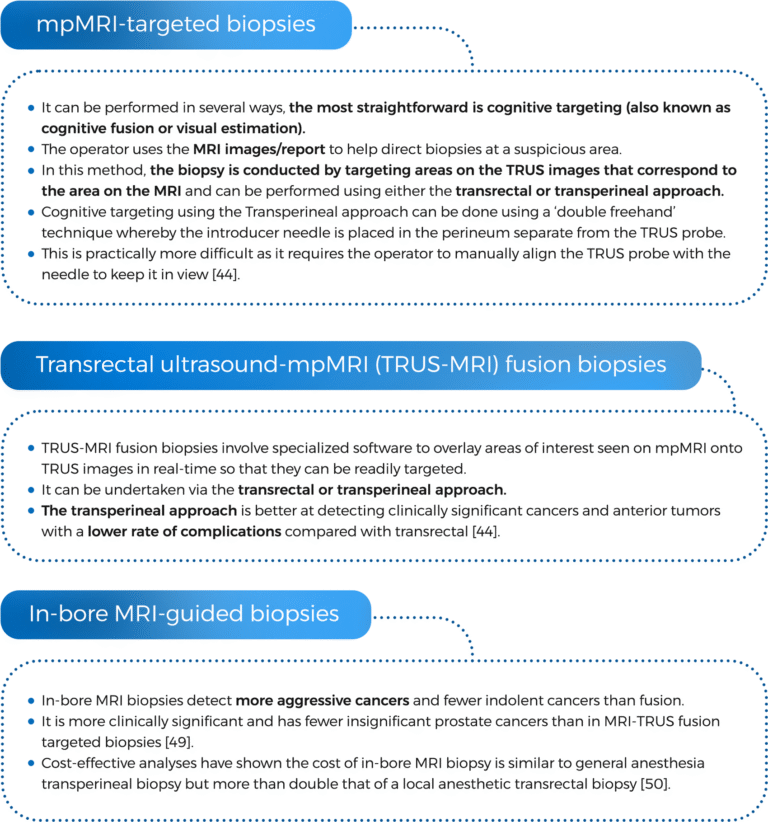
44. Paul Gravestock, B., et al., Prostate cancer diagnosis: Biopsy approaches. Exon Publications, 2022: p. 141-168.
45. Grups, J.W., et al., Diagnostic Value of Transrectal Ultrasound in Tumor Staging and in the Detection of Incidental Prostatic Cancer. Urologia Internationalis, 2010. 45(1): p. 38-40.
46. Zani, E.L., O.A. Clark, and N. Rodrigues Netto, Jr., Antibiotic prophylaxis for transrectal prostate biopsy. Cochrane Database Syst Rev, 2011(5): p. Cd006576.
47. Hodge, K.K., et al., Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. The Journal of urology, 1989. 142(1): p. 71-74.
48. Mottet, N., et al., EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. European Urology, 2021. 79(2): p. 243-262.
49. Costa, D.N., et al., Magnetic Resonance Imaging-guided In-bore and Magnetic Resonance Imaging-transrectal Ultrasound Fusion Targeted Prostate Biopsies: An Adjusted Comparison of Clinically Significant Prostate Cancer Detection Rate. Eur Urol Oncol, 2019. 2(4): p. 397-404.
50. Altok, M., et al., Cost and efficacy comparison of five prostate biopsy modalities: a platform for integrating cost into novel-platform comparative research. Prostate Cancer and Prostatic Diseases, 2018. 21(4): p. 524-532.
51. Barral, M., et al., In-Bore Transrectal MRI–Guided Biopsy With Robotic Assistance in the Diagnosis of Prostate Cancer: An Analysis of 57 Patients. American Journal of Roentgenology, 2019. 213(4): p. W171-W179.
52. Chen, N. and Q. Zhou, The evolving Gleason grading system. Chin J Cancer Res, 2016. 28(1): p. 58-64.
53. cancer, Z.p. Staging of prostate cancer. Available from: https://zerocancer.org/stages-and-grading#risk.
54. foundation, U.c. Prostate staging Available from: https://www.urologyhealth.org/urology-a-z/p/prostate-cancer.
55. (CDC), C.f.D.C.a.P. Prostate cancer treatment Available from: https://www.cdc.gov/prostate-cancer/treatment/index.html.
56. Institute, N.C. Prostate Cancer Treatment (PDQ®)–Health Professional Version. Available from: https://www.cancer.gov/types/prostate/hp/prostate-treatment-pdq.
57. (NHS), N.h.S.U. Prostate cancer treatment Available from: https://www.nhs.uk/conditions/prostate-cancer/treatment/.
58. research, C. Prostate cancer treatment. Available from: https://www.cancerresearchuk.org/about-cancer/prostate-cancer/treatment.
59. clinic.org, C. Prostate cancer managment and treatment Available from: https://my.clevelandclinic.org/health/diseases/8634-prostate-cancer#management-and-treatment.
60. center, J.H. Proton Therapy for Prostate Cancer. Available from: https://www.hopkinsmedicine.org/health/conditions-and-diseases/prostate-cancer/proton-therapy-for-prostate-cancer#:~:text=Proton%20beam%20therapy%20for%20prostate%20cancer%20is%20a%20noninvasive%20external,and%20organs%20from%20radiation%20exposure.
61. Brogden, R.N. and P. Chrisp, Flutamide. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in advanced prostatic cancer. Drugs Aging, 1991. 1(2): p. 104-15.
62. Schellhammer, P.F. and J.W. Davis, An Evaluation of Bicalutamide in the Treatment of Prostate Cancer. Clinical Prostate Cancer, 2004. 2(4): p. 213-219.
63. Beer, T.M., et al., Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. New England Journal of Medicine, 2014. 371(5): p. 424-433.
64. Patel, U.J. and S. Caulfield, Apalutamide for the Treatment of Nonmetastatic Castration-Resistant Prostate Cancer. J Adv Pract Oncol, 2019. 10(5): p. 501-507.
65. Yamaoka, M., et al., Orteronel (TAK-700), a novel non-steroidal 17, 20-lyase inhibitor: effects on steroid synthesis in human and monkey adrenal cells and serum steroid levels in cynomolgus monkeys. The Journal of steroid biochemistry and molecular biology, 2012. 129(3-5): p. 115-128.
66. He, Y., et al., Targeting signaling pathways in prostate cancer: mechanisms and clinical trials. Signal Transduction and Targeted Therapy, 2022. 7(1): p. 198.
67. Taplin, M.-E., et al., Androgen receptor modulation optimized for response—Splice variant: A phase 3, randomized trial of galeterone versus enzalutamide in androgen receptor splice variant-7–expressing metastatic castration-resistant prostate cancer. European urology, 2019. 76(6): p. 843-851.
68. Madan, R.A., et al., Phase 2 Study of Seviteronel (INO-464) in Patients With Metastatic Castration-Resistant Prostate Cancer After Enzalutamide Treatment. Clin Genitourin Cancer, 2020. 18(4): p. 258-267.e1.
69. Goulooze, S.C., A.F. Cohen, and R. Rissmann, Olaparib. Br J Clin Pharmacol, 2016. 81(1): p. 171-3.
70. Institute, N.C. With Two FDA Approvals, Prostate Cancer Treatment Enters the PARP Era. 2020; Available from: https://www.cancer.gov/news-events/cancer-currents-blog/2020/fda-olaparib-rucaparib-prostate-cancer.
71. FDA. FDA approves olaparib with abiraterone and prednisone (or prednisolone) for BRCA-mutated metastatic castration-resistant prostate cancer. 2023; Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-olaparib-abiraterone-and-prednisone-or-prednisolone-brca-mutated-metastatic-castration#:~:text=On%20May%2031%2C%202023%2C%20the,mCRPC)%2C%20as%20determined%20by%20an.
72. de Bono, J.S., et al., Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): an open-label, phase 2 trial. The Lancet Oncology, 2021. 22(9): p. 1250-1264.
73. FDA. FDA approves talazoparib with enzalutamide for HRR gene-mutated metastatic castration-resistant prostate cancer. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-enzalutamide-hrr-gene-mutated-metastatic-castration-resistant-prostate.
74. Handy, C.E. and E.S. Antonarakis, Sipuleucel-T for the treatment of prostate cancer: novel insights and future directions. Future Oncol, 2018. 14(10): p. 907-917.
75. Prostate cancer prevention. Available from: https://www.pcf.org/patient-resources/family-cancer-risk/prostate-cancer-prevention/.
All Rights Reserved to Indigoarabia.com
Stay Connected for latest Genitourinary news
connect with us on LinkedIn for professional updates and collaborations.
All Rights Reserved to Indigoarabia.com